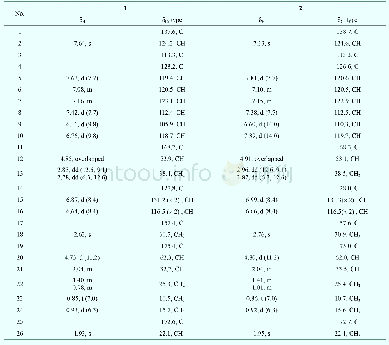
《Table 1 1H NMR and 13C NMR Data for compounds 1 and 2a》
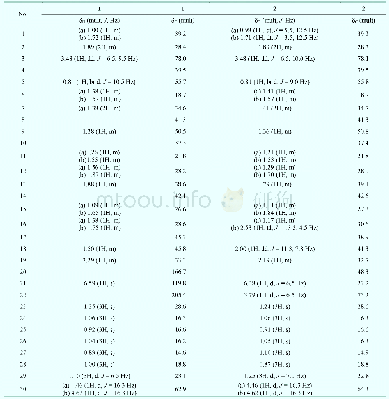 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Taraxastane-type triterpenoids from the medicinal and edible plant Cirsium setosum》
a Data were recorded on a Bruker Avance 500 spectrometer.Chemical shifts(δ)are given in parts per million with references to the center peak of C5D5Nwithδ7.20 for 1H andδ123.44 for 13C.*Data can be interchanged.
Compound 1 was obtained as a white amorphous powder.Its molecular formula C30H48O3,with 7 degrees of unsaturation,was indicated by HREIMS at m/z 456.3638(M+-)(Calcd.456.3603) .The IR spectrum of 1 suggested that it contained hydroxyl groups(3438 and 3338 cm–1)and conjugated carbonyl(1655 cm–1)functionalities.The 1H NMR data(Table 1)of 1 in C5D5N displayed signals for six methyl singlet groups(δH 0.89,0.92,1.00,1.04,1.06 and 1.25),a methyl doublet group[δH 1.10(3H,d,J=6.5 Hz)],one oxygenated methine[δH 3.48(1H,dd,J=6.5,9.5 Hz)],one oxygenated methylene[δH 4.62(1H,d,J=16.3 Hz)and 4.46(1H,d,J=16.3 Hz)]and a trisubstituted olefinic proton[δH 6.59(1H,s)].The 13C NMR spectrum displayed 30 carbon signals,which were classified as seven methyls,nine methylenes(one oxygenated),seven methines(one oxygenated and one olefinic carbon),and seven quaternary carbons(one carbonyl and one olefinic carbons)on the basis of DEPT and HMQC spectra.These data suggested that 1 was similar with one known taraxastane-type triterpenoid,22-oxo-20-taraxasten-3β-ol[11],except for an additional hydroxyl group located at C-30,which was confirmed by the comprehensive analysis of the 2D NMR spectra of 1,especially 1H-1H COSY and HMBC(Fig.2).
| 图表编号 | XD0047580800 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.01.20 |
| 作者 | LIN Peng-Cheng、JI Lin-Lin、ZHONG Xiang-Jian、LI Jin-Jie、WANG Xin、SHANG Xiao-Ya、LIN Sheng |
| 绘制单位 | College of Pharmaceutical Sciences,Qinghai University for Nationalities、Beiijing Key Laboratory of Bioactive Substances and Functional Foods,Beijing Union University、Beiijing Key Laboratory of Bioactive Substances and Functional Foods,Beijing Union Univer |
| 更多格式 | 高清、无水印(增值服务) |