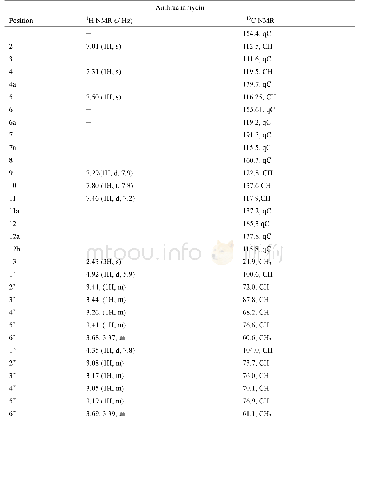
《Table 1 1H (400 MHz) and 13C NMR (100 MHz) data for compound 1 (in DMSO-d6)》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《"Romipeptides A and B, two new romidepsin derivatives isolated from Chromobacterium violaceum No.968 and their antitumor activities in vitro"》
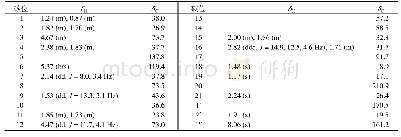
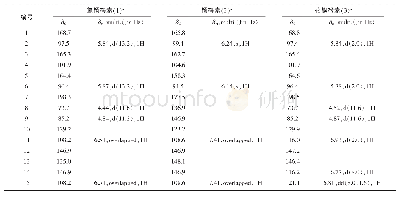
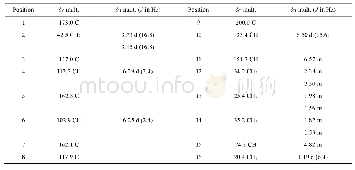
Compound 1 was obtained as a colorless crystal,with the molecular formula of C48H72O12N8S4 based on HR-ESI-MS,1H NMR and 13C NMR data.Compared with the molecular formula(C24H36O6N4S2)of compound 4,compound 1 might be the dimer of compound 4.The 13C NMR and DEPT data of compound 1(Table 1)revealed the presence of 24 carbon signals,including one quaternary carbon,five carbonyl carbons,five methyl carbons,four methylene carbons and nine methine carbons,which were identical to those of compound 4.Based on above analysis,the structure of compound 1 might be symmetrical.The 13C NMR signals of compound 1(Table 1)were similar to those of compound 4,except the signals for C-20(C-20'),C-16(C-16'),C-11(C-11'),C-10(C-10')and C-9(C-9'),which related to disulfide bonds.By comparison of the13C NMR data of compounds 1 and 4,chemical shift values for C-11(C-11')and C-16(C-16')were very different withδC52.3 andδC 41.4 presented in compound 1,whileδC 56.6 andδC 35.2 presented in compound 4,respectively.The carbon C-16 was directedly connected at the disulfide bond and the carbon C-11 was connected at C-16 in compound 4.Based on the above analysis,the disulfide bonds of compound 1 might be formed between two molecules of compound 4.The structure of compound 1 was further deduced by comprehensive interpretation of its 1H-1H COSY and HMBC spectra(Fig.2).
| 图表编号 | XD0062385000 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.02.20 |
| 作者 | XIONG Lei、CHEN Chang-Fa、MIN Tao-Ling、HU Hai-Feng |
| 绘制单位 | State Key Lab of New Drug&Pharmaceutical Process, Shanghai Institute of Pharmaceutical Industry, China State Institute of Pharmaceutical Industry、State Key Lab of New Drug&Pharmaceutical Process, Shanghai Institute of Pharmaceutical Industry, China State |
| 更多格式 | 高清、无水印(增值服务) |