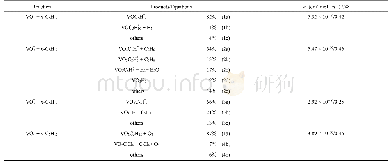
《Table 1The H2-evolution rate and quantum efficiency (QE) of the various sam-ples.》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Ni纳米粒子作为电子转移剂和NiS_x作为产氢界面活性位协同增强TiO_2光催化制氢性能(英文)》
To confirm that Ni and NiSx promote the H2-evolution reaction,the photoelectrocatalytic performance of various samples was investigated.Using LSV measurements(Fig.9 (A)) ,the Ti O2/Ni sample was found to show a lower overpotential than the TiO2 sample owing to the fast transfer of electrons by metallic Ni.For the TiO2/Ni-Ni Sx(30%)sample,the overpotential dramatically decreases,suggesting that Ni Sx forms better interfacial active sites than metallic Ni,thus providing excellent kinetics for efficiently reducing H+to H2.However,the Ti O2/Ni Sx sample displays a slightly increased overpotentia compared with the TiO2/Ni-Ni Sx(30%)sample.This difference can be attributed to the lack of an electron-transfer mediator in the TiO2/Ni Sx sample,resulting in a slow rate of electron transfer that affects the subsequent interfacial H2-evolution reaction In fact,efficient photocatalytic H2-evolution materials require not only rapid electron transfer but also an effective interfacial catalysis reaction for H2 production.Therefore,both the metallic Ni electron-transfer cocatalyst and the NiSx interfacial active sites are important for the H2-evolution reaction of TiO2.Furthermore,the transient photocurrent responses(Fig.9 (B)) and electrochemical impedance spectra(Fig.9 (C)) of the Ti O2/Ni-Ni Sx(30%)sample exhibit the highest photocurrent density and the smallest arc radius,respectively,among the various samples,indicating that this sample has the highest separation efficiency of photogenerated carriers.According to the above results,it can be concluded that the excellent synergistic effect of metallic Ni nanoparticles as electron-transfer cocatalysts and Ni Sx as interfacial active sites in the Ti O2/Ni-Ni Sx system contributes to its enhanced photocatalytic H2-evolution activity.
| 图表编号 | XD0028865700 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.03.01 |
| 作者 | 王苹、徐顺秋、陈峰、余火根 |
| 绘制单位 | 武汉理工大学化学化工与生命科学学院化学系、武汉理工大学化学化工与生命科学学院化学系、武汉理工大学化学化工与生命科学学院化学系、武汉理工大学化学化工与生命科学学院化学系、武汉理工大学硅酸盐建筑材料国家重点实验室 |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 1The H2-evolution rate and quantum efficiency (QE) of the various sam-ples.”的人还看了
-
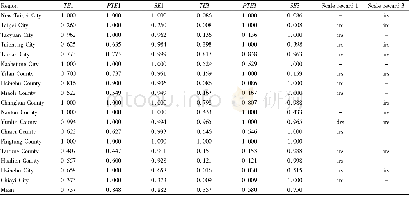
- Table 1 Comprehensive technical efficiency, pure technical efficiency and scale efficiency of agricultural production in