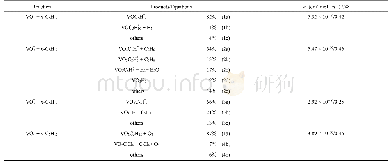
《Table 1The degradation rates and k values for RhB (phenol) degradation by as-prepared samples and o
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《缺陷辅助表面修饰提高g-C_3N_4@C-TiO_2直接Z型异质结的可见光光催化性能(英文)》
The photocatalytic activities of the pure Ti O2,g-C3N4g-C3N4@Ti O2,g-C3N4@5C-Ti O2,g-C3N4@10C-Ti O2,and g-C3N4@20C-Ti O2 samples were examined for their Rh B and phenol photodegradation ability under visible-light irradiation(Fig.6).The g-C3N4@C-Ti O2 sample exhibited higher photocatalytic degradation efficiency than those of the pure Ti O2g-C3N4,and g-C3N4@Ti O2 samples(without carbon).With increasing carbon content,the photocatalytic degradation efficiency of g-C3N4@C-Ti O2 initially increased and subsequently decreased.Calculation of the Langmuir-Hinshelwood mechanism(shown in Fig.6 (b)and(d)) showed that g-C3N4@10C-Ti O2 exhibited the largest apparent constant(k)of0.036 and 0.039 min-1 for RhB and phenol photodegradation respectively,which was 150/139,6.4/6.8,2.3/3.0,and 1.7/2.1times higher than those of pure Ti O2,10C-Ti O2,g-C3N4,and g-C3N4@Ti O2,respectively.For comparison,the degradation rates and k values for Rh B and phenol degradation by the as-prepared samples and other reported photocatalysts are listed in Table 1[35–38].
| 图表编号 | XD0028866300 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.03.01 |
| 作者 | 李喜宝、熊杰、许英、冯志军、黄军同 |
| 绘制单位 | 南昌航空大学材料科学与工程学院、南昌航空大学材料科学与工程学院、湖南科技大学物理与电子科学学院、南昌航空大学材料科学与工程学院、南昌航空大学材料科学与工程学院 |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 1The degradation rates and k values for RhB (phenol) degradation by as-prepared samples and other reported photoca”的人还看了
-
- Table 1 Products, pseudo-first-order rate coefficients (k) , and reaction efficiencies (Φ) for the reactions investigate
-

- 表1 高碳铬铁冶炼主要元素分配率设定值%Tab.1The set value of major elements allocation rate for high carbon ferrochrome production
-
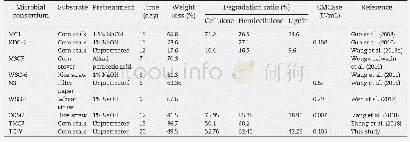
- Table 5–Comparison for the weight loss, degradation ratio and cellulase activities (CMCase) of TC-Y with that of various





