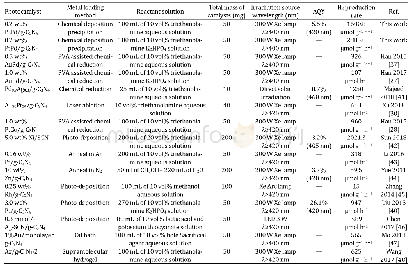
《Table 1Comparison of photocatalytic H2 evolution of metal/g-C3N4 photocatalysts reported in the lit
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《新型PtPd合金纳米颗粒修饰g-C_3N_4纳米片以提高可见光照射下光催化产氢活性(英文)》
The photocatalytic H2 evolution activities of the prepared samples were characterized and the results are shown in Fig.5.The pure g-C3N4 sample shows a relatively low H2 production rate of 2.2μmol g–1 h–1.After loading the PtPd alloy NPs onto the surface of the g-C3N4 nanosheets,the photocatalytic activity of the sample for hydrogen production was significantly enhanced.After loading only 0.1 wt%PtPd alloy NPs,the rate of H2 production was remarkably increased to 1207.1μmol g–1h–1:nearly 600 times higher than that of pure g-C3N4.Along with the gain from increase of the PtPd alloy NP weight ratios,the H2 evolution activity of the PtPd/g-C3N4 composite sample was also improved when the weight fraction of the PtPd alloy was increased to 0.2 wt%.The 0.2 wt%Pt Pd/g-C3N4 sample gave the highest H2 evolution activity(rate of 1600.8μmol g–1h–1),800 times higher than that of pure g-C3N4.It can be seen that deposition of the PtPd alloy NPs can remarkably improve the hydrogen evolution activity of pure g-C3N4.However,further increase in the deposition ratio of PtPd alloy NPs to higher than 0.2 wt%results in a decrease in the H2 production rate The degraded photocatalytic H2 evolution activity might be caused by the light shielding effect of the excess black Pt Pd alloy NPs,which would attenuate the light intensity at the surface of the g-C3N4.To explore the conditions needed to provide the greatest potential of the PtPd/g-C3N4 composite sample for photocatalytic H2 evolution,K2HPO4 was added to the electrolyte as a sacrificial agent.When 10 g of K2HPO4 was added to the photocatalytic system,the H2 production rate of the Pt Pd/g-C3N4 composite sample reached 2885.0μmol g–1 h–1The explanation of this enhancement is that HPO42–can act as a mediator that directly takes part in the photocatalytic H2 production[27,40].From Table 1,it can be noted that better hydrogen production rates were obtained in this study than with the other monometallic and bimetallic photocatalysts reported in the literature.
| 图表编号 | XD0028865800 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.03.01 |
| 作者 | 肖楠、李松松、刘霜、徐博冉、李延东、高旸钦、戈磊、卢贵武 |
| 绘制单位 | 中国石油大学(北京)重质油国家重点实验室、中国石油大学(北京)新能源与材料学院、中国石油大学(北京)新能源与材料学院、中国石油大学(北京)新能源与材料学院、中国石油大学(北京)新能源与材料学院、中国石油大学(北京)新能源与材料学院、中国石油大学(北京)新能源与材料学院、中国石油大学(北京)重质油国家重点实验室、中国石油大学(北京)新能源与材料学院、中国石油大学(北京)新能源与材料学院 |
| 更多格式 | 高清、无水印(增值服务) |