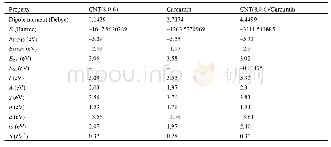
《Table 3 Substrate scope of complex Ni Cl2L4 (4) catalyzed the C—C coupling Reactions》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《2-吡唑啉-9-芳基邻菲咯啉镍配合物催化芳基卤化物和格氏试剂偶联反应的研究(英文)》
a.R and X mean substituents in aryl halides.The reactions were carried out using 1 mol%4,aryl halides 1.0 mmol,PhMgBr 1.2 mmol for 24 h at 20℃in 5 m L toluene;b.GC yield
With the optimized conditions in hand,the scope of aryl halides was investigated(Table 3).For example,complex 4 acted as an efficient catalyst for the substrateof p-MeC6H4I,reaching 90%yield within 24 h at room temperature(Table 3,entry 6).Under same conditions,when p-CF3C6H4I as a substrate gave excellent conversations(yield up to 98%,Table 3,entry 7).On the same experimental conditions,complex 4 also applies to deactivated substrate such as 4-ioideanisol,but the yield of the corresponding product is a bit lower,reaching 63%yields for deactived aryl iodides over a period of 24 h(Table 3,entry 5).Probably because of the nature of the substituents(electron-withdrawing or electron-donating substituents)in the aromatic substrates,which play a key role on the activation of C—I bboonnddss..UUnnffoorrttuunnaatteellyy,,wwhheenn ddeeaaccttiivvaatteedd ssuubbssttrraatteess 44--bromotoluene and 4-bromoanisole were involved,only moderate or trace amounts of products were observed.In expectation,due to low reactivity of aryl chlorides,nickel complexes are almost inefficient even for the coupling reaction of activated 4-chlorotoluene and PhMgBr.It is easily explain that C—Cl bond(Ph—X bond dissociation energies (BDEs)[34]:Cl,402 k J/mol;Br,339 kJ/mol;I,272 kJ/mol) much stronger.In addition,the steric hindrance of the substituent has little influence on the product yield in the C—C coupling reaction.
| 图表编号 | XD0018900300 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.10.01 |
| 作者 | 崔凤娟、王环宇、邓庆芳、江艳、曹艳珍、赵桦萍 |
| 绘制单位 | 齐齐哈尔大学化学与化学工程学院、齐齐哈尔大学化学与化学工程学院、齐齐哈尔大学化学与化学工程学院、齐齐哈尔大学化学与化学工程学院、齐齐哈尔大学化学与化学工程学院、齐齐哈尔大学化学与化学工程学院 |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 3 Substrate scope of complex Ni Cl2L4 (4) catalyzed the C—C coupling Reactions”的人还看了
-

- 表2 基材与Ni-Cr-B-Si涂层阻抗谱拟合数据Table 2 Impedance spectrum fitting parameters of substrate and Ni-Cr-B-Si coating