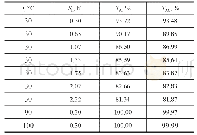
《Table 2Substrate scope of olefins.》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《脯氨酸衍生的胺基吡啶四氮锰配合物催化双氧水参与的烯烃不对称环氧化反应(英文)》
Reaction conditions:substrate(0.4 mmol),C1(0.2 mol%),CH3CN as a solvent(0.5 m L),DMBA(0.5 eq.),and 30%H2O2(1.5 eq.)in 0.5 m LMe CN was added dropwise via a syringe pump over 30 min with stirring at–30°C and then further stirring for 30 min.
The activities of three structurally new catalysts were then examinedinasymmetricepoxidation.Weselected cis-β-methylstyrene as a model substrate and used2,2-dimethylbutanoic acid(DMBA)as the additive[26].Epoxidation was performed in CH3CN at–30°C using 30%aqueous H2O2(1.5 eq.)as the oxidant.It was shown that the catalyst C1(0.1 mol%)was the most effective,furnishing epoxide in 84%yield and 82%ee(Table 1,entry 1).Surprisingly,the manganese catalyst C2,with a larger steric hindrance,exhibited decreased performance despite its richer electronic properties(Table 1,entry 2).In addition,the manganese complex C3,with fewer electron-donating d MM groups,exhibited lower yield and enantioselectivity under the same conditions(Table 1,entry 3).To improve the conversion in epoxidation,a larger catalyst loading(0.2 mol%)was tested,and it was found that the substrate converted completely,and a 92%yield and 88%ee were obtained(Table 1,entry 4).The reaction proceeded well even in the presence of 50 mol%DMBA when 0.2 mol%manganese complex C1 was used(Table 1,entry 5).
| 图表编号 | XD008193800 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.09.01 |
| 作者 | 王文芳、孙强盛、夏春谷、孙伟 |
| 绘制单位 | 中国科学院兰州化学物理研究所羰基合成与选择氧化国家重点实验室、中国科学院大学、中国科学院兰州化学物理研究所羰基合成与选择氧化国家重点实验室、中国科学院兰州化学物理研究所羰基合成与选择氧化国家重点实验室、中国科学院兰州化学物理研究所羰基合成与选择氧化国家重点实验室 |
| 更多格式 | 高清、无水印(增值服务) |