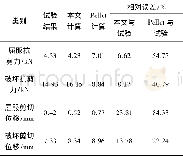
《Table 2 Comparison of experiment value and value calculated by kinetic model of olefin conversion1)
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Kinetic Model of Hydrogenation for Removal of Trace Olefins from Alkylation Mixture Formed during Linear Alkylbenzene Synthesis》
1) Hydrogenation conditions covered:a temperature of 30°C and a hydrogen partial pressure of 1.0 MPa.
The hydrogenation reaction system included three phases consisting of gas,liquid,and solid catalyst.At the lower reaction temperature,the saturated vapor pressure of hydrocarbons was far less than the reaction pressure and the hydrogen partial pressure was close to the reaction pressure.It was assumed that the gas phase only contained hydrogen,and the hydrogen partial pressure was equal to the reaction pressure.The hydrogenation experiments for removal of trace olefins were carried ou in a fixed bed reactor to investigate the effects of WHSV hydrogen partial pressure,and reaction temperature on olefin conversion.The experimental results under different conditions are listed in Table 1.The results in Table 1 showed that the conversion increased with the decrease of WHSV(SF),and the increase in hydrogen partial pressure or reaction temperature.This might occur because the increase of the hydrogen partial pressure or the reaction temperature both could increase the reaction rate.Reducing the weight hourly space velocity of the hydrogenation feedstock could prolong the contact time with the catalyst bed,which was advantageous to the improvement in olefins conversion rate.
| 图表编号 | XD0029186300 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.03.30 |
| 作者 | Ren Jie、Liu Bing、Shen Lian |
| 绘制单位 | College of Chemical Engineering, Zhejiang University of Technology、College of Chemical Engineering, Zhejiang University of Technology、College of Chemical Engineering, Zhejiang University of Technology |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 2 Comparison of experiment value and value calculated by kinetic model of olefin conversion1)”的人还看了
-
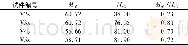
- 表6 试件受弯承载力计算值与试验值对比Table 6 Comparison on the calculated values and tested values of the specimens’fexure capacity