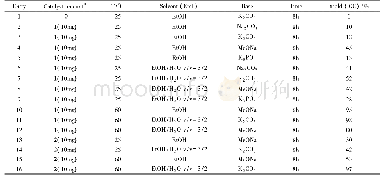
《Table 4 Knoevenagel condensation reaction catalyzed by complex 1*》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《"[Zn(Pybox)Br_2]配合物的合成,晶体结构及催化性质研究(英文)"》
*Isolated yield;Reaction condition:activated methylene compound(0.5 m M,2.0 equiv);ferrocenecarboxaldehyde(0.25 m M),1 mol%Zn catalyst,and 1.0m L H2O,under air.
Knoevenagel condensationreaction between ferrocenecarboxaldehyde and active methylene compounds is often used to test the catalytic activity of obtained zinc complex 1.The ferrocenecarboxaldehyde and different active methylene compounds were performed to give the desired products in high yields using 1 mol%zinc complex 1catalyst in water at room temperature(Table 4,2a,2b,2c and 2d).The results showed that the complex(DmPybox)Zn Br2was more efficient than that of the complex(Dm-Pybox)Zn Cl2[13].The difference in catalytic activity is most likely due to the bromide anion being a better leaving group compared to chloride anion.Knoevenagel condensation reactions are more likely to take place when bromide anion replace with chloride anion in zinc complexes with Dm-pybox ligand.
| 图表编号 | XD00411700 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.05.01 |
| 作者 | 支雪婷、朱孟增、贾卫国 |
| 绘制单位 | 安徽师范大学化学与材料科学学院、安徽师范大学化学与材料科学学院、安徽师范大学化学与材料科学学院 |
| 更多格式 | 高清、无水印(增值服务) |