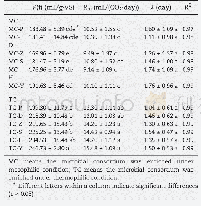
《Table 2 Oligomerizations activated by the reaction products of C6H5OH/4-t BuPhOH, AlMe3 and water a
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Preparation of Aryloxy-aluminoxanes and Their Use as Activators in the Bis(imino)pyridyl Iron-catalyzed Oligomerization of Ethylene》
a General conditions:pre-cat.:L-Fe(acac)3,[Fe]=2μmol;[Al]/[Fe]=2000;solvent:toluene,50 mL;T=50°C;P=0.1 MPa;t=30 min;b The mass fraction of insoluble polymers in the total products;c Total activity:kg/ (mol Fe·h)
To further verify the activation effectiveness of the aryloxyaluminoxanes for iron-catalyzed ethylene oligomerization,unsubstituted phenol(C6H5OH)and para-alkyl substituted phenol(4-t BuPhOH)were also employed to prepare the correspondingphenoxy-aluminoxanes.Hereby,the[H2O]/[Al]molar ratio used in the hydrolysis procedure was fixed at 0.7.As shown in Table 2,these aluminoxanes also serve as highly effective activators;the activities and the polymer-retarding abilities are close to those of the 4-Brphenoxy-aluminoxanes.As expected,the[―OH]/[Al]molar ratio adopted in the first step of reaction with AlMe3has very significant impacts on the catalytic activity,olefin distribution,and most importantly the polymer formation.There is a sharp reduction of the polymer yields as the[―OH]/[Al]ratio increases from 0.1 to 0.7.In these cases,[―OH]/[Al]=0.5 continues to be the optimized value,which can largely retard the polymer production and maintain the activity at a high level.
| 图表编号 | XD0020171800 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.11.01 |
| 作者 | Wei Zhang、Bin-Bo Jiang、Jian Ye、Zu-Wei Liao、Zheng-Liang Huang、Jing-Dai Wang、Yong-Rong Yang |
| 绘制单位 | Zhejiang Provincial Key Laboratory of Advanced Chemical Engineering Manufacture Technology, State Key Laboratory of Chemical Engineering, College of Chemical and Biological Engineering, Zhejiang University、Zhejiang Provincial Key Laboratory of Advanced Ch |
| 更多格式 | 高清、无水印(增值服务) |