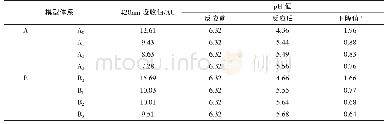
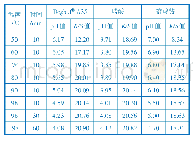
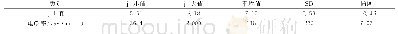《表1 不同pH值和反应时间对应的样品组成》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《BiVO_4纳米结构的形貌和组成控制及其对光催化性能的影响(英文)》
Based on above photocatalytic measurements,the sample at pH=7 has superior photocatalytic performance over other three samples.Herein,the mechanism of the enhanced photocatalytic activity can be understood as described below.In Fig.8(a),it is analyzed that the sample at pH=7 has the narrowest bandgap and the highest visible absorption,thus more photons will be absorbed and then more electron-hole pairs will be excited,leading to the enhancement of the photocatalytic activity.More importantly,the control of morphologies also plays a crucial role in the adjustment of photocatalytic activities.The sample at pH=7has very special morphology.Nanoscale BiVO4particles were self-assembled and accumulated on the porous central core,and resultantly,mesoporous nanostructures with large specific surface area were constructed.The smaller particle size,which means shorter diffusion length of the charge carrier,is beneficial to overcome the limitations of BiVO4such as short carrier lifetime and low mobility,and promote the separation of electron-hole pairs.Moreover,the sample at pH=7 has the largest specific surface area,which will induce high density surface state sites.The existence of the surface state enhances the surface adsorption,suppresses the recombination in bulk,and lengthens the carrier lifetime[30].In addition,the special morphology of the mesoporous structure,large surface area and small particle size can increase the contact area with water,provide more active sites and capture more photoexcited carriers.It is also noted that the sample at pH=7 has the best dispersion in the MB solution,which can be ascribe to the special morphology such as small particle size and large surface area.This suspension behavior reduced the transmission and scattered loss of incident light(e.g.reflection)in the solution that enhanced the incident photon energy per unit surface area of the photocatalyst,accelerating the improvement of the photocatalytic activity.For the sample at pH values of 3 and 5,larger particle size,smaller surface area as well as narrower bandgap result in relative weak photocatalytic performance,and the sample at pH=5 has a relative higher photocatalytic performance compared to the sample at pH=3.Finally,the tubular BiVO4structures in the sample at pH=9 are composed of irregular long rod-like crystals(longer than10μm).The largest crystal size as well as the compact aggregation lead to the poor dispersion,the smallest surface area and the resultant poor photocatalytic activity.
| 图表编号 | XD00145026800 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2020.06.15 |
| 作者 | 印川、施天宇、赵晨媛、尹海宏、宋长青、秦琳、王志亮、邵海宝 |
| 绘制单位 | 南通大学信息科学技术学院、南通大学信息科学技术学院、南通大学信息科学技术学院、南通大学信息科学技术学院、南通大学信息科学技术学院、南通大学信息科学技术学院、南通大学信息科学技术学院、南通大学信息科学技术学院 |
| 更多格式 | 高清、无水印(增值服务) |