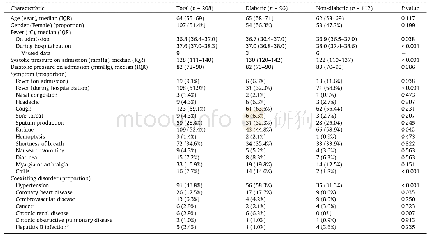
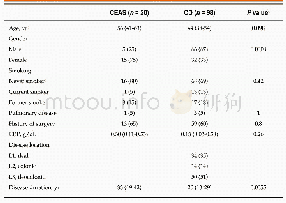
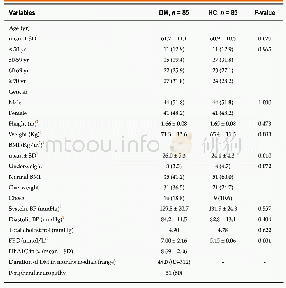
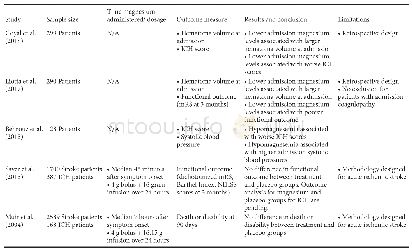
《Table 1 Clinical studies about pembrolizumab》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《PD-1/PD-L1 pathway blockade works as an effective and practical therapy for cancer immunotherapy》
So far,the FDA has approved five drugs that target the PD-1/PD-L1 pathway for cancer treatment.These five drugs are Keytruda(pembrolizumab),Opdivo(nivolumab),Bavencio(avelumab),Tecentriq(atezolizumab),and Imfinzi(durvalumab).Pembrolizumab,nivolumab,and durvalumab are PD-1 antibodies,while atezolizumab and avelumab are PD-L1 antibodies.The clinical studies that gained the FDAapproval of pembrolizumab are listed in Table 1.As shown in Table 1,pembrolizumab has been approved for the treatment of seven different types of cancer.Particularly,the approval of pembrolizumab for the treatment of microsatellite instability high(MSI-H)or mismatch repairdeficient(d MMR)solid tumors is the first time that the FDAhas approved a drug for cancer treatment based on the marker rather than the location of cancer origin,which also reveals the extensive applicability of cancer immunotherapy.
| 图表编号 | XD008421400 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.05.01 |
| 作者 | Long Jia、Qi Zhang、Rongxin Zhang |
| 绘制单位 | Laboratory of Immunology and Inflammation,Department of Immunology and Research Center of Basic Medical Sciences,Key Laboratory of Immune Microenvironments and Diseases of Educational Ministry,Tianjin Medical University、Institute of Integrative Medicines |
| 更多格式 | 高清、无水印(增值服务) |