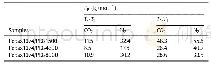
《Table 1Calculated lowest activation energies for the first (EH1) and second C–H bonds (EH2) of prop
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《VO_3/CeO_2(111)催化剂上丙烷氧化脱氢反应活性和选择性的密度泛函理论研究(英文)》
In Table 1,we list the calculated barriers for the first and second C–H bond breaking processes discussed above.We also list the calculated results of the same processes for propane and ethane on V2O5(001)reported in previous studies by Fan and co-workers[45,46].As it can be seen from the table,the calculated reaction barriers for both the first and second C–H bond breaking processes on the clean V2O5(001)are quite high.More significantly,the first C–H bond typically appears to be more difficult to break than the second one for both propane and ethane;this is in agreement with the regular order of the bond strengths.Most interestingly,from our calculations,we can find that on the supported VO3/Ce O2(111)system,the first C–H bond breaking step is actually very easy to occur,which has an activation barrier of only 0.12 e V.In contrast,the second C–H bond breaking step is relatively significantly difficult with a barrier of 1.81 e V.To better understand the reason for the reversed activities toward C–H bond activation in alkanes,we conducted further electronic analyses for this interesting catalyst.
| 图表编号 | XD008196700 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.09.01 |
| 作者 | 黄昶、王志强、龚学庆 |
| 绘制单位 | 华东理工大学化学与分子工程学院计算化学中心结构可控先进功能材料及其制备教育部重点实验室、华东理工大学化学与分子工程学院计算化学中心结构可控先进功能材料及其制备教育部重点实验室、华东理工大学化学与分子工程学院计算化学中心结构可控先进功能材料及其制备教育部重点实验室 |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 1Calculated lowest activation energies for the first (EH1) and second C–H bonds (EH2) of propane at VO3/Ce O2 (111”的人还看了
-
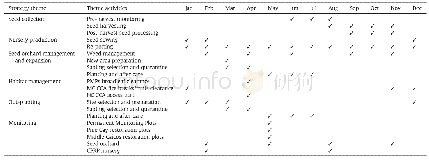
- Table AAnnual Activity Implementation Table for the Caicos Pine Restoration Strategy.PMPs-Permanent Monitoring Plots;MC
-

- Table 2.Activation energies of permeation for CO2and N2through Pebax1074 mem-brane and its blend membranes with low mole