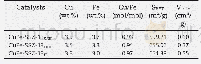
《Table 1Physical‐chemical properties of xβ‐25Cu/Si O2 catalysts.》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《碳酸乙烯酯选择加氢合成甲醇与乙二醇高效稳定Cu/SiO_2催化剂研究(英文)》
a Determined by ICP‐AES.b Determined by N2O titration.c Obtained by N2 physisorption.
Fig.2 presents N2 adsorption‐desorption isotherms of a se‐ries of xβ‐25Cu/Si O2 catalysts;the corresponding textural properties are also summarized in Table 1.The actual copper loadings were determined by ICP‐AES,and the results showed that the actual Cu contents were essentially close to the nomi‐nal value.The N2 physisorption isotherms of xβ‐25Cu/Si O2catalysts exhibited type IV isotherms with an H1‐type hystere‐sis loop[34]indicating that the mesoporous structures were formed.Table 1 shows that the unmodified 25Cu/Si O2 had a surface area of 242 m2 g–1 with an average pore size of 5.2 nm.In contrast,the surface areas of xβ‐25Cu/Si O2(x=2,5,8)were326–351 m2 g–1,and the values gradually declined with in‐creasingβ‐cyclodextrin content.These results indicated that the surface area of 25Cu/Si O2 could be markedly enhanced viaβ‐cyclodextrin.Meanwhile,the average pore sizes of the xβ‐25Cu/Si O2 catalysts were primarily distributed in the larger pore regions(5.5–7.5 nm).This is beneficial for mass transfer during the reaction process and could provide abundant easily accessible active sites for hydrogenation.N2O titration indicat‐ed that the introduction of an appropriate amount ofβ‐cyclodextrin could enhance the copper dispersion.Specifi‐cally,the maximal metallic Cu surface area of Cu/Si O2 was 26.8m2 g–1 with a high Cu dispersion of 28.6%.Nevertheless,the introduction of excessiveβ‐cyclodextrin,i.e.8%,obviously de‐creased the metallic Cu0 surface area(19.7 m2 g–1).
| 图表编号 | XD008190400 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.08.01 |
| 作者 | 刘佳驹、贺鹏、王利国、刘辉、曹妍、李会泉 |
| 绘制单位 | 中国科学院过程工程研究所中国科学院绿色过程与工程重点实验室、北京化工大学、中国科学院过程工程研究所中国科学院绿色过程与工程重点实验室、中国科学院过程工程研究所中国科学院绿色过程与工程重点实验室、盐城工学院江苏省生态建材与环保装备协同创新中心、中国科学院大学中丹学院、北京化工大学、中国科学院过程工程研究所中国科学院绿色过程与工程重点实验室、中国科学院过程工程研究所中国科学院绿色过程与工程重点实验室、中国科学院大学中丹学院 |
| 更多格式 | 高清、无水印(增值服务) |