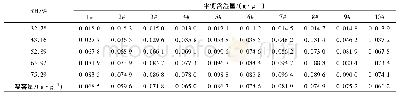
《Table 2XPS results for the Co Cu/Ti O2 and x‐Co Cu/Ti O2 catalysts after reduction.》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《碱金属助剂对CoCu/TiO_2催化剂上二氧化碳加氢合成长链烃的影响(英文)》
The metal content in the catalyst was derived from the XRF measurements and is listed in Table 1.As can be seen from Table 1,the content of alkali metals and the Co/Cu molar ratio were in close agreement with the nominal compositions used for preparing the catalysts,indicating that there were no obvi‐ous metal losses.Additionally,the added alkali metals may exist in the form of carbonates under the reduction conditions at 350°C due to the much higher decomposition temperature of car‐bonates,which can be seen from the TG curves of those sam‐ples(Fig.S1).The Li NO3 promoter may decompose into an oxide during the reduction process(350°C),while lithium ox‐ide would be easily converted to carbonate under a CO2 at‐mosphere because the Gibbs free energy for this reaction is negative.
| 图表编号 | XD008191600 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.08.01 |
| 作者 | 石志彪、杨海艳、高鹏、陈新庆、刘洪江、钟良枢、王慧、魏伟、孙予罕 |
| 绘制单位 | 上海大学理学院化学系、中国科学院上海高等研究院中科院低碳转化科学与工程重点实验室、中国科学院上海高等研究院中科院低碳转化科学与工程重点实验室、中国科学院上海高等研究院中科院低碳转化科学与工程重点实验室、中国科学院上海高等研究院中科院低碳转化科学与工程重点实验室、上海大学理学院化学系、中国科学院上海高等研究院中科院低碳转化科学与工程重点实验室、上海科技大学物质学院、中国科学院上海高等研究院中科院低碳转化科学与工程重点实验室、中国科学院上海高等研究院中科院低碳转化科学与工程重点实验室、上海科技大学物质学院、中 |
| 更多格式 | 高清、无水印(增值服务) |