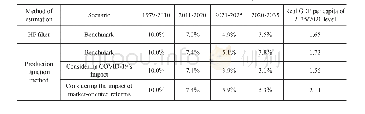
《Table 1.Deposition potentials (vs.CO32-/CO2–O2) of carbon via reaction (9) and alkali metals via re
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Capture and electro-splitting of CO_2 in molten salts》
The values in Table 1 were calculated by HSC software according to the Nernst equation[23].
The potentials of reactions(9)and(10)are listed in Table 1,which shows that in molten Li2CO3,carbon deposition is thermodynamically preferred to Li deposition,however,when comparing Na2CO3and K2CO3,the metal is the thermodynamically preferred component for deposition.These results imply that Li2CO3is an essential ingredient in pure metal carbonates for carbon deposition[25,34].
| 图表编号 | XD0042897000 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.01.01 |
| 作者 | Wei Weng、Lizi Tang、Wei Xiao |
| 绘制单位 | School of Resource and Environmental Sciences, Hubei International Scientific and Technological Cooperation Base of Sustainable Resource and Energy,Wuhan University、School of Resource and Environmental Sciences, Hubei International Scientific and Technolo |
| 更多格式 | 高清、无水印(增值服务) |