《Table 3Catalytic performance for CO2 hydrogenation over the x‐Co Cu/Ti O2 catalysts.》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《碱金属助剂对CoCu/TiO_2催化剂上二氧化碳加氢合成长链烃的影响(英文)》
Reaction conditions:p=5.0 MPa,T=250°C,WHSV=3000 m L·gcat–1·h–1,H2/CO2/N2=73/24/3.a Yield C5+=CCO2Stotal hydrocarbonSC5+.
The catalytic performances of the alkali‐metal‐promoted catalysts were investigated to study the effect of the alkali promoters under the given reaction conditions.For compari‐son,the pure Co Cu/Ti O2 catalyst was tested under the same reaction conditions.The catalytic activities and hydrocarbon selectivities are listed in Table 3.The products are mainly CO and hydrocarbons,and the selectivity of the oxy‐compound is not listed because it was less than 0.5%when the reaction temperature was higher than 230°C for the Co Cu/Ti O2 and Na‐Co Cu/Ti O2 catalysts(Table S2).The cobalt catalyst was normally used in FTS applications for its higher chain growth probabilities[23,32].However,when the feed gas was changed to CO2 and H2,the main product over the pure Co Cu/Ti O2 cata‐lyst was CH4.The RWGS reactivity was very low,and the CO selectivity was only 1%for the Co Cu/Ti O2 catalyst,as can be seen in Table 3.Therefore,it reacted as a methanation catalyst rather than as an FT catalyst under the reaction conditions.
| 图表编号 | XD008191400 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.08.01 |
| 作者 | 石志彪、杨海艳、高鹏、陈新庆、刘洪江、钟良枢、王慧、魏伟、孙予罕 |
| 绘制单位 | 上海大学理学院化学系、中国科学院上海高等研究院中科院低碳转化科学与工程重点实验室、中国科学院上海高等研究院中科院低碳转化科学与工程重点实验室、中国科学院上海高等研究院中科院低碳转化科学与工程重点实验室、中国科学院上海高等研究院中科院低碳转化科学与工程重点实验室、上海大学理学院化学系、中国科学院上海高等研究院中科院低碳转化科学与工程重点实验室、上海科技大学物质学院、中国科学院上海高等研究院中科院低碳转化科学与工程重点实验室、中国科学院上海高等研究院中科院低碳转化科学与工程重点实验室、上海科技大学物质学院、中 |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 3Catalytic performance for CO2 hydrogenation over the x‐Co Cu/Ti O2 catalysts.”的人还看了
-

- Table 5 Input parameters for alternator design based on the performance evaluation of horizontal axis wind turbine torqu
-
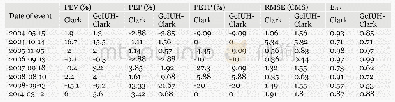
- Table 8 The values of performance criteria for Clark and GcIUH-Clark models for different rainfall-runoff events
-

- Table 1 Distance dH—Xbetween the hydrogen atom and the adsorbed atoms X (X=N for NH3, O for H2O, O for NO2) *





