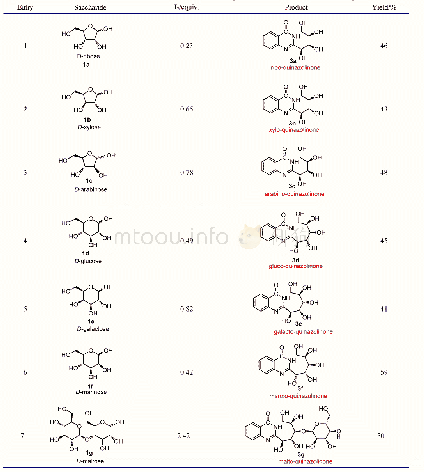
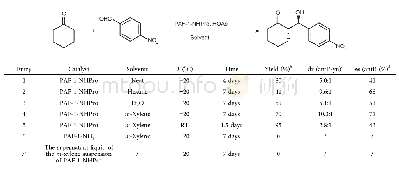
《Table 1 The control experiments for PAF-1-NHPro catalyzed Aldol reactiona》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《以多孔芳香骨架材料PAF-1作为对映选择性有机催化的超稳定固载平台(英文)》
a) Conditions:a mixture of p-nitrobenzaldehyde(0.25 mmol),catalyst(27 mg),HOAc(0.2 mmol)and cyclohexanone(2.5 mmol)in solvents(0.5 mL)was stirred at the indicated temperature for the indicated time.b) The isolated yield of the mixture of the an
With the expected PAF-1-NHPro in hand,its catalytic performance was evaluated,adopting Aldol reaction between p-nitrobenzaldehyde and cyclohexanone as a model reaction.Because the quantity of the immobilized catalytic site was difficult to accurately calculate for PAF-1-NHPro,the catalyst loading was selected by an easy initial investigation and then remained unchanged in the control experiments as indicated in Table 1.This is different from the research on the homogeneous catalysis.First,we screened various solvents(neat,hexane,Et2O,m-xylene)and m-xylene gave the best results in terms of diastereoselectivity,enantioselectivity and yield of the current catalytic reaction(entry 1–4,Table 1).So we used m-xylene as the solvent to further investigate our heterogeneous catalysis.The elevation of reaction temperature from-20°C to room temperature increased the reactivity of the current reaction but seriously reduced the enantioselectivity and diastereoselectivity(entry 5,Table 1).In addition,the material PAF-1-NH2(entry 6,Table 1)could not catalyze the current reaction under the optimized conditions,indicating that the introduced chiral prolinamide unit in PAF-1-NHPro is indeed the effective catalytic site for the current reaction.The supernatant liquid of the m-xylene suspension of PAF-1-NHPro had no catalytic activity(entry 7,Table 1),which definitely indicated no leakage of catalytically active species from the PAF-1-NHPro catalyst during the catalysis process.It is noteworthy that the operation after reaction is very simple,in which only centrifugation is needed to remove the solid catalyst and the obtained solution could be further directly purified by evaporation and flash column chromatography to yield the desired product.
| 图表编号 | XD0039959700 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.02.01 |
| 作者 | 陈鹏、孙金时、张蕾、马文悦、孙福兴、朱广山 |
| 绘制单位 | State Key Laboratory of Inorganic Synthesis and Preparative Chemistry, College of Chemistry, Jilin University、Institute of Drug Discovery Technology, Ningbo University、State Key Laboratory of Inorganic Synthesis and Preparative Chemistry, College of Chemi |
| 更多格式 | 高清、无水印(增值服务) |