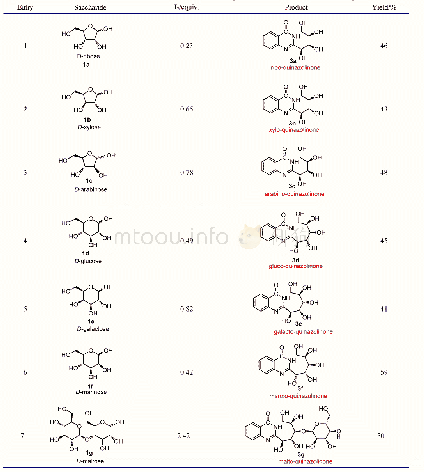
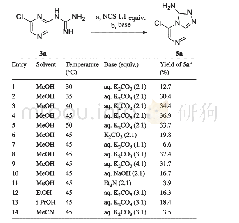
《Table 2 Synthesis of the aldo-quinazolinones 3a~3g through the condensation of the unprotected sacc
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《碘诱导的醛糖与o-氨基苯甲酰胺的氧化缩合合成糖基喹唑啉酮(英文)》
We initiated our experiment by examining the reaction of D-ribose(1a)with o-aminobenzamide(2a)in dimethyl sulfoxide(DMSO)solution at 60℃(Table 1,Entry 1)according to the method for the aromatic aldehydes.[13]However,the reaction didn’t occur.Subsequently,the reaction was carried out in methanol solution at 40℃in the presence of some Lewis acids Fe Cl3[19]or CuCl2[20](Table1,Entries 2,3),but the reactions still didn’t proceed.When using Ac OH as the catalyst,[33]the reaction could yield small amount of the intermediate A(Scheme 1,two pairs of inseparable amino glycosides includingα/β-furanosides andα/β-pyranosides)(Table 1,Entry 4).Finally,iodine(I2)was added to promote the reaction(Table 1,Entry 5).[21]The reaction could succeed in generating the target ribo-quinazolinone 3a with a low yield of 12%,although the iodine-induced reaction was very complicated.
| 图表编号 | XD00212952700 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.07.01 |
| 作者 | 琚欢欢、孙佳婧、李小六、陈华 |
| 绘制单位 | 河北大学化学与环境科学学院河北省化学生物学重点实验室、河北大学化学与环境科学学院河北省化学生物学重点实验室、河北大学化学与环境科学学院河北省化学生物学重点实验室、河北大学化学与环境科学学院河北省化学生物学重点实验室 |
| 更多格式 | 高清、无水印(增值服务) |

![Table 3 Cyclization reaction in the synthesis of[1, 2, 4]triazolo[4, 3-a]pyrazin-3-amines 5](http://bookimg.mtoou.info/tubiao/gif/TJDY201902009_09800.gif)