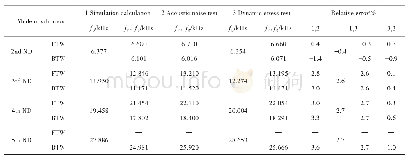
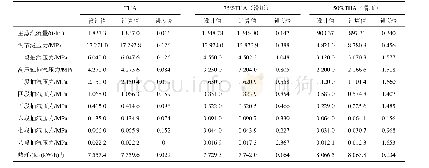
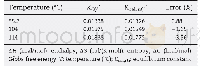
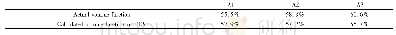《Table 4–Comparison between the calculated (Kcal, (3) ′′) and experimental (K (3) ′′) derived revers
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Thermodynamics of NaHCO_3 decomposition during Na_2CO_3-based CO_2 capture》
As expected,the Kcal,(1) values were much larger than Kcal,(3) at higher temperatures(above 75°C)because the heat required to initiate the reaction is not lost to the solution in the case of solid decomposition(Tables 1 and 2).Thus,the decomposition of sodium bicarbonate in an aqueous solution cannot happen as easily as it does in solid decomposition.
| 图表编号 | XD0033524400 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.04.15 |
| 作者 | Sam Toan、William O'Dell、Christopher K.Russell、Sun Zhao、Qinghua Lai、Huiping Song、Ying Zhao、Maohong Fan |
| 绘制单位 | College of Engineering and Applied Science,University of Wyoming、Chemical and Environmental Engineering,University of California、College of Engineering and Applied Science,University of Wyoming、Civil and Environmental Engineering,Stanford University、Colle |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 4–Comparison between the calculated (Kcal, (3) ′′) and experimental (K (3) ′′) derived reverse reaction data at di”的人还看了
-

- 表4 2组Scr、BUN、e GFR、ADMA比较 (±s, n=40) Tab.4 Comparison of Scr, BUN, e GFR and ADMA between the two groups