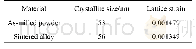《Table 2Dispersion and particle size of Ru NPs, as determined by CO chemisorption and TEM, for Ru‐MC
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《以RuCl_3/SiO_2为模板制备高性能镶嵌式钌基氨合成催化剂(英文)》
a Determined via EDSb Dem=(1-NsNc)100%,where Ns refers to the number of metal atoms present on the surface,as measured by CO chemisorption,and Nc refers to the number of metal atoms present on the surface,based on the average crystallite size deter
The status of Ru NPs was examined by HRTEM.It is known that the Ru NPs supported on AC are difficult to be observed by TEM owing to the influence of C,especially in the cases of cata‐lysts with small nanoparticles(less than 2 nm).The HRTEM images of Ru‐MC at different resolutions are given in Fig.3(d?f).For a comparison,the TEM image of Ru/MC is provided in Fig.3(c).The black dots distributed in the HRTEM images are Ru particles.It can be seen that the Ru NPs both in Ru‐MC and Ru/MC are uniformly distributed on the supports and no ag‐gregation is observed.The particle size distributions of Ru‐MC(Fig.3 (e)) and Ru/MC(Fig.3 (c)) are both in the range 1.5–2.5nm,and the average particle size is ca.2 nm.Fig.S2 shows the STEM images and particle size distributions of Ru NPs for Ba‐K/Ru‐MC and Ba‐Ru‐K/MC catalysts.It can be seen that the particle size distributions of the abovementioned catalysts are relatively narrow.The average particle size of Ba‐K/Ru‐MC is2.2 nm,whereas that of Ba‐Ru‐K/MC is 2.3 nm(Table 2),which is almost the same as those of the Ru‐MC and Ru/MC samples.As is known,ammonia synthesis is a structure‐sensitive reac‐tion.The most active sites for N2 dissociation and ammonia synthesis are ensembles of five Ru atoms,named B5‐type sites[28].Jacobsen et al.[19]reported that the B5 sites are only present on Ru particles larger than 0.7 nm.The probability of existence of B5 sites is maximum for Ru particles of size 1.8–2.5nm,and monotonically decreases for particles larger than 2.5nm.Therefore,if only the particle size is considered,both the Ba‐K/Ru‐MC and Ba‐Ru‐K/MC catalysts with Ru particles in the size range 1.5–2.5 nm are appropriate for forming more active B5‐type sites.From Fig.3(f),it can be seen that most of the Ru NPs on the Ru‐MC catalyst are semi‐embedded in the mesopo‐rous C framework.This phenomenon has been observed in Ru‐OMC catalysts as well and has been fully discussed[13–15].Uniform Ru NPs were obtained by thermal reduction;the con‐sumption of C during the reduction of Ru3+to Ru enables the Ru NPs to sit in or semi‐embed in the C matrix.To estimate the degree of embedding of the Ru NPs,the dispersion of Ru parti‐cles was characterized by CO chemisorption for the Ru‐MC and Ru/MC catalysts,and the results are included in Table 2.The dispersion of Ru for Ru‐MC is 35.2%,while it is 65.6%for Ru/MC,according to the CO chemisorption measurements.The CO monolayer uptakes of Ru‐MC and Ru/MC are 138.4 and258.0μmol/g,respectively.Based on the chemisorption data and assuming the adsorption of one CO per metal atom,one can estimate the exposed surface area of the metal catalysts.It can be seen that the exposed active Ru surface areas are 5.1 and 9.5m2/g.On the other hand,the Ru NPs sizes of Ru‐MC and Ru/MC obtained by HRTEM are almost the same.This indicates that a fraction of the Ru NPs are embedded in the C matrix.The de‐gree of embedding was calculated by the formula Dembedding=(1-Ns?Nc)?100%,where Ns refers to the number of metal at‐oms present on the surface,as measured by CO chemisorption,and Nc refers to the number of metal atoms present on the sur‐face,based on the average crystallite size determined by HRTEM.The estimated degrees of embedding of the Ru NPs are given in Table 2.It can be seen that the degree of embedding of Ru‐MC is 47.1%,whereas it is 1.4%for Ru/MC,thus indicating that the Ru NPs of Ru‐MC are semi‐embedded in the C struc‐ture.However,the dispersions of Ru in the Ba‐K/Ru‐MC and Ba‐Ru‐K/MC catalysts are 36.2%and 29.4%,respectively.This may be due to the addition of promoters,which cover some of the Ru NPs and,thereby,affect the dispersion of the catalysts.It can be considered that the degrees of embedding of Ba‐K/Ru‐MC and Ba‐Ru‐K/MC do not represent the state of existence of the Ru NPs on the C surface.
| 图表编号 | XD0028864700 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.01.01 |
| 作者 | 周亚萍、马永承、蓝国钧、唐浩东、韩文锋、刘化章、李瑛 |
| 绘制单位 | 浙江工业大学工业催化研究所、四川省华地建设工程有限责任公司、浙江工业大学工业催化研究所、浙江工业大学工业催化研究所、浙江工业大学工业催化研究所、浙江工业大学工业催化研究所、浙江工业大学工业催化研究所、浙江工业大学工业催化研究所 |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 2Dispersion and particle size of Ru NPs, as determined by CO chemisorption and TEM, for Ru‐MC and Ru/MC.”的人还看了
-

- 表2 粒径大小对复合粉结块率及感官品质的影响Table 2 Effect of particle size on caking rate and sensory quality of composite powder
-

- 表3 粒径大小对复合粉结块率及感官品质的影响Table 3 Effect of particle size on caking rate and sensory quality of composite powder
-

- Table 1–Effect of different concentrations of propranolol on zeta potential and particle size of the nanoparticles (mean