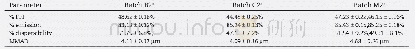
《Table 1 Characterization of PMMA macroinitiators initiated by TsCl or EBPA》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Effect of Halogen Chain End Fidelity on the Synthesis of Poly(methyl methacrylate-b-styrene) by ATRP》
a[Initiator]/[CuX]/[bpy]/[MMA]/[anisole]=1/1/2/600/600;b[Initiator]/[CuX]/[bpy]/[MMA]=1/1/2/1200;c Measured by GPC using THF as eluent based on PMMA standards.All reactions were carried out at 60°C.
In order to compare the effect of the halogen chain end on the synthesis of PMMA-b-PS,EPBA and TsCl were chosen as the initiators to synthesize bromide/chloride terminated PMMA-Br and PMMA-Cl macroinitiators because they showed similar initiation rates under the same polymerization conditions and the resulting PMMAs had similar molecular weights and narrow PDIs(Scheme 1a)[22,23].The polymerization was performed both in bulk and in anisole solution and the target number-average molecular weight(Mn)was~50 kg/mol.The polymerization time and the conversion of MMA monomers were very close when the target Mn of PMMA was the same for the EPBA/CuBr/bpy and TsCl/CuCl/bpy initiation systems in bulk(Table 1).However,when the polymerization was performed in anisole solutions,a shorter polymerization time was needed to achieve the same molecular weight for the EPBA/CuBr/bpy system.Compared to the TsCl/CuCl/bpy system,the monomer conversion was also lower for the EPBA/CuBr/bpy system,but it was proportional to the polymerization time.A lower monomer conversion required for the same molecular weight using theEPBA/CuBr/bpy system might be caused by a higher coupling rate.These two sets of data showed that the polymerization of MMA using the EPBA/CuBr/bpy and TsCl/CuCl/bpy initiation systems had similar polymerization rates.
| 图表编号 | XD0020172000 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.11.01 |
| 作者 | Guang-Cheng Huang、Sheng-Xiang Ji |
| 绘制单位 | Key Laboratory of Polymer Ecomaterials, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences、University of Chinese Academy of Sciences、Key Laboratory of Polymer Ecomaterials, Changchun Institute of Applied Chemistry, Chinese Academy of Sc |
| 更多格式 | 高清、无水印(增值服务) |