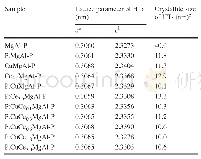
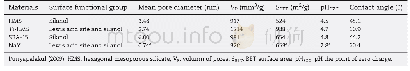
《Table S3Comparison of the OER activity for the synthesized coral‐like Ni Fe (OH) x/Ni with several
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《通过温和的镍腐蚀制备珊瑚状FeNi(OH)_x/Ni作为一种一体化高效水分解电极(英文)》
*The data were i R corrected accordingly.
The OER catalytic performance of Fe Ni(OH)x/Ni,coral‐like Ni,Ru O2/Ni,and Fe Ni Ox/Ni was evaluated by LSV measure‐ments in 1.0 mol L-1 KOH solution using a standard three‐electrode configuration.As shown in Fig.4(a),prior to oxygen evolution,prominent oxidation waves were observed for both Fe Ni(OH)x/Ni and coral‐like Ni,which can be assigned to the redox couple of Ni2+/Ni3+[47].This observed oxidation peak shifted to a higher potential over Fe Ni(OH)x/Ni,which indicates the occurrence of charge transfer from Ni to Fe in Fe/Ni hydroxides,as demonstrated in the literature[48,49].Apparently,Fe Ni(OH)x/Ni showed the highest OER activity,as revealed by the polarization curves.For example,for Fe Ni(OH)x/Ni,an overpotential of only 254 m V was required to reach a current density of 50 m A cm-2,whereas for Fe Ni Ox/Ni,Ru O2/Ni,and coral‐like Ni,the overpotentials were as high as338,390,and 443 m V,respectively.Furthermore,a similar activity trend was found after normalizing the activity to the electrochemical surface area(ECSA)(Fig.S9(a)) .Correspond‐ingly,the kinetic activities of all the electrodes were assessed using Tafel plots derived from the polarization curves.As shown in Fig.4(b)and Table S1,the smallest Tafel slope was acquired over Fe Ni(OH)x/Ni(45 m V dec-1).In order to avoid the arbitrary influence of replotting the polarization curve to calculate the Tafel slope[10],an analysis method based on electrochemical impedance spectroscopy(EIS)was also con‐ducted(Fig.S10),and a more comprehensive value of 42.8 m V dec-1 was obtained.Such excellent catalytic activity makes Fe Ni(OH)x/Niamongthebestreportednonpre‐cious‐metal‐based OER catalysts(Table S3).
| 图表编号 | XD008197900 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.11.01 |
| 作者 | 向锐、童成、王尧、彭立山、聂瑶、李莉、黄寻、魏子栋 |
| 绘制单位 | 重庆大学化学化工学院清洁能源与资源利用化学过程重点实验室、重庆大学化学化工学院清洁能源与资源利用化学过程重点实验室、重庆大学化学化工学院清洁能源与资源利用化学过程重点实验室、重庆大学化学化工学院清洁能源与资源利用化学过程重点实验室、重庆师范大学化学学院、重庆大学化学化工学院清洁能源与资源利用化学过程重点实验室、重庆大学化学化工学院清洁能源与资源利用化学过程重点实验室、重庆大学化学化工学院清洁能源与资源利用化学过程重点实验室 |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table S3Comparison of the OER activity for the synthesized coral‐like Ni Fe (OH) x/Ni with several recently reported hig”的人还看了
-
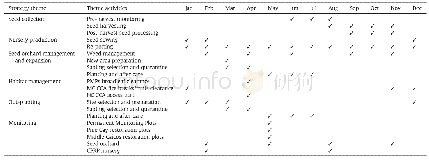
- Table AAnnual Activity Implementation Table for the Caicos Pine Restoration Strategy.PMPs-Permanent Monitoring Plots;MC