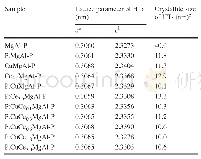
《Table 1–Physicochemical characteristics of the synthesized adsorbents.》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Adsorption of single and mixed haloacetonitriles on silica-based porous materials: Mechanisms and effects of porous structures》
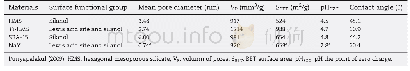
The XRD patterns of the as used HMS,Ti-HMS,SBA-15 and NaY samples are shown in Fig.1.HMS exhibited the highest corresponding hexagonal porous structure with a clear(100)plane at 2θ=2.2o.A shift in the(100)plane to a higher 2θangle was observed for the Ti-HMS,which can be attributed to a partial collapse of the HMS framework during the adsorbent synthesis.For SBA-15,three diffraction peaks were clearly revealed at 2θ=0.92o,1.58oand 1.84o,which correspond to the(100),(110) and(200)planes,respectively,reflecting the welldefined hexagonal array of the mesopore.The standard peaks of the NaY were 6o,10o,12o,15o,18o,20o,24o,27oand31o(Fig.1).The patterns attained from the synthesized SBA-15and the commercial NaY seen here are similar to those previously reported(Aguado et al.,2009;Sue-aok et al.,2010Tao et al.,2010;Wei et al.,2005).The determined BET surface area,pore diameter and pore volume data,based on the N2adsorption–desorption isotherms(Fig.2),are summarized in Table 1 including the previously reported data from these same sample preparations.Additionally,the XPS technique has been used to investigated the surface chemical environment of HMS(Fig.3).The survey spectrum of HMS reveals that the main elements of sample were Si and O(Si 2p at 104.6 e V and O 1 s at 533.3 eV,respectively).SEM micrographs of HMS and SBA-15 are shown in Fig.4.HMS exhibited an aggregated spherical particles,which is consistent with the report of Punyapalakul et al.(2009).Moreover,SBA-15 presented continuous rod-shaped structure.
| 图表编号 | XD0052063900 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.05.15 |
| 作者 | Panida Prarat、Chawalit Ngamcharussrivichai、Sutha Khaodhiar、Patiparn Punyapalakul |
| 绘制单位 | International Postgraduate Programs in Hazardous Substance and Environmental Management, Graduate School, Chulalongkorn University、Center of Excellence on Hazardous Substance Management (HSM), Chulalongkorn University、Department of Chemical Technology, Fa |
| 更多格式 | 高清、无水印(增值服务) |