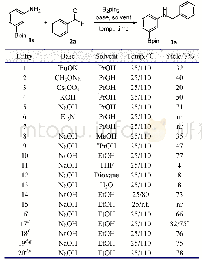
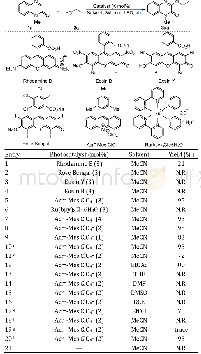
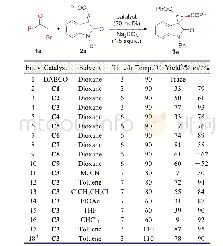
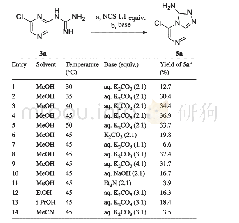
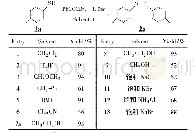
《Table 1 Optimization of the reaction conditionsa)》
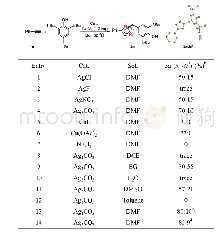 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《"Silver-catalyzed three-component reaction: synthesis of N~2-substituted 1,2,3-triazoles via direct benzylic amination"》
a) Reaction conditions:1a(0.5 mmol),2a(0.6 mmol.),NaN3(0.6 mmol),Cat.(0.3 eq.),Sol.(1 mL),50°C,6 h.b) Isolated yield.c) 2.0eq.NaHCO3was added.d) 2.0 eq.KF was added.DMF=N,N-dimethylformamide;DCE=1,2-dichloroethane;EG=ethylene glycol;DMSO
Based on our continuous interest in alkyne reactions,our investigation began with the reaction of two easily accessible phenylacetylene 1a and BHT 2a as the model substrates to verify our hypothesis of the extensive screening of transition-metal catalysts and solvents(Table 1 and Supporting Information online).Gratifyingly,we could obtain the desired isomeric triazole 3a with 65%yield in 3.3:1 ratio of 3aN2and 3a-N1by employing AgCl as the catalyst and DMF as the solvent(Table 1,entry 1).The two isomers were clearly confirmed by single-crystal X-ray analysis[18a]of 1,4-disubstituted product 3a-N1(see Supporting Information online),assisted by the13C chemical shifts of the triazole CH[19].Guided by this exciting result,other silver salts such as AgF,AgNO3and Ag2CO3were then examined using model substrates for 1,2,3-triazoles by in-situ C(sp3)–H/N–H crosscoupling(Table 1,entries 2–4).A good yield of product 3a was achieved with Ag2CO3as catalyst under air atmosphere.Diminished or no catalytic activity was exhibited when the reaction was performed with other commercially available metal sources such as CuI,Cu(OAc)2,NiCl2(Table 1,entries5–7).The click/coupling tandem reaction employing DCE,EG,H2O,DMSO and toluene as solvent was investigated and furnished 3a in the unsatisfactory outcome(Table 1,entries 8–12).We further examined base as additives in the tandem reaction(Table 1,entries 13,14).KF was proved to be beneficial for the reaction and delivered 1,2,3-triazole product 3a in the highest yield with a ratio of N2-substituted/N1-substituted(N2/N1)1,2,3-triazoles up to 9.1:1.
| 图表编号 | XD0066337600 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.08.01 |
| 作者 | Zhenhua Liu、Wenjing Hao、Wen Gao、Guangyu Zhu、Xiang Li、Lili Tong、Bo Tang |
| 绘制单位 | College of Chemistry,Chemical Engineering and Materials Science,Collaborative Innovation Center of Functionalized Probes for Chemical Imaging in Universities of Shandong,Key Laboratory of Molecular and Nano Probes,Ministry of Education,Shandong Provincial |
| 更多格式 | 高清、无水印(增值服务) |