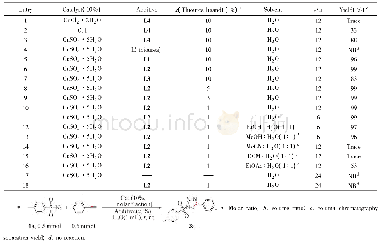
《Table 1Optimization of the reaction conditions a,b.》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《可见光催化喹喔啉-2(1H)-酮与醇C(sp~2)-H/O-H交叉脱氢偶联反应(英文)》
Our preliminary exploration started from the photocatalytic CDC reaction of N‐methylquinoxalin‐2(1H)‐one(1a)as the template substrate,ethanol(3 equiv.,2a)as the coupling rea‐gent,and 3 mol%of Rhodamine B as the photocatalyst.When the reaction mixture was irradiated by a 3W blue LED lamp(465 nm)in acetonitrile under air exposure for 12 h,the ex‐pected 3‐ethoxy‐N‐methylquinoxalin‐2(1H)‐one(3aa)was generated in 21%NMR yield(Table 1,entry 1).Inspired by this experimental result,various photocatalysts were screened(entries 2-6).Among these examined photocatalysts,9‐mesityl‐10‐methylacridinium(Acr+‐Mes Cl O4‐)was the opti‐mal catalyst,furnishing product 3aa in 95%yield(entry 5).Varying the loading of the catalyst(entries 7–9)revealed that the Acr+‐Mes Cl O4?loading could be reduced to 2 mol%without any impact on the yield of 3aa(entry 8).Next,the screening of the amount of Et OH did not afford better results(entries10–11).A series of solvents was investigated(entries 12–16),and the results showed that the solvents played a pivotal role in this transformation.Only in acetonitrile and ethyl acetate did the reaction occur smoothly(entries 8 and 12).Other reaction media,such as THF,DMF,DMSO,and DCE,did not support the visible‐light photocatalytic CDC reaction(entries 13-16).Changing MeCN to EtOH resulted in the production of the ex‐pected product(3aa)in a 75%yield(entry 17).When the model reaction was performed under irradiation by the 3Wgreen LED lamp or sunlight,only trace or 16%yield of the de‐sired product(3aa)was observed,respectively(entries 18 and19).The reaction with dioxygen as an oxidant did not provide a better result(entry 20).No CDC reaction was observed in the absence of Acr+‐Mes Cl O4?(entry 21).
| 图表编号 | XD00146367700 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2020.08.01 |
| 作者 | 谢龙勇、刘艺舒、丁红汝、龚绍峰、谭家希、何军意、曹忠、何卫民 |
| 绘制单位 | 湖南科技学院化学学院、湖南科技学院化学学院、湖南科技学院化学学院、湖南科技学院化学学院、湖南科技大学化学化工学院、长沙理工大学电力与交通材料保护实验室、长沙理工大学电力与交通材料保护实验室、湖南科技学院化学学院 |
| 更多格式 | 高清、无水印(增值服务) |
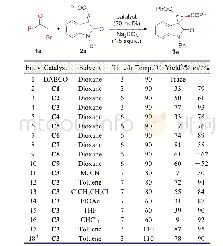
![Table 3 Cyclization reaction in the synthesis of[1, 2, 4]triazolo[4, 3-a]pyrazin-3-amines 5](http://bookimg.mtoou.info/tubiao/gif/TJDY201902009_09800.gif)