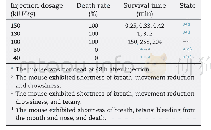
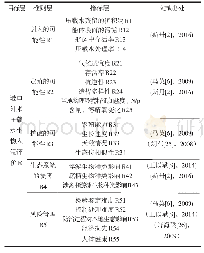
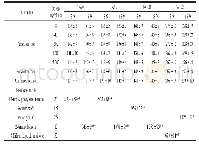
《Table 1Toxicity assessment of B.thuringiensis 29.118 to mice by bacterial reverse mutation assay》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《传统发酵食品臭鳜鱼中分离的苏云金芽孢杆菌(Bacillus thuringiensis 29.118)的毒理学评价(英文)》
Note:**.significantly different from the solvent control group(P<0.01).
The toxicity assessments of B.thuringiensis for mammals have aroused great public concerns recently.The bacterial reverse mutation assay is used to assess the safety of specific chemicals as an effective method of mutagenic evaluation.In the present study,the mutagenic effects of the B.thuringiensis 29.118 were evaluated by the bacterial reverse mutation assays of four S.typhimurium strains(TA97,TA98,TA100 and TA102).The experimental results showed that no distinct evidence of mutagenicity was found;compared to the solvent control,there were no significant differences in the numbers of revertants with four S.typhimurium strains following treatment with concentration gradients(8,40,200,1 000 and 5 000μg per plate)(Table 1) .The experiment of positive control meant the tested results were reliable,and the given gradients of concentration could be confirmed that no mutagenic evidence was found using the bacterial reverse mutational assay.Each group with and without S-9 rat liver had significant differences compared to the positive chemical.
| 图表编号 | XD0041253800 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.02.15 |
| 作者 | 杨培周、朱星星、操丽丽、郑志、程杰顺、姜绍通 |
| 绘制单位 | 合肥工业大学食品与生物工程学院安徽省农产品精深加工省级实验室、合肥工业大学食品与生物工程学院安徽省农产品精深加工省级实验室、合肥工业大学食品与生物工程学院安徽省农产品精深加工省级实验室、合肥工业大学食品与生物工程学院安徽省农产品精深加工省级实验室、合肥工业大学食品与生物工程学院安徽省农产品精深加工省级实验室、合肥工业大学食品与生物工程学院安徽省农产品精深加工省级实验室 |
| 更多格式 | 高清、无水印(增值服务) |