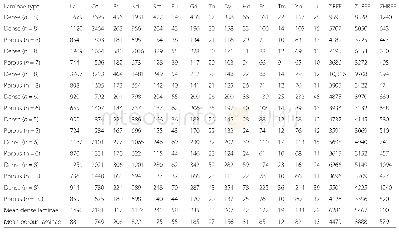
《Table 2Thermal properties of the copolymer produced over rare‐earth‐metal ternary catalyst with var
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《稀土三元催化体系ZnO/SiO_2负载化及季铵盐催化CO_2与环氧丙烷合成高分子量聚碳酸酯(英文)》
a Onset of degradation with respect to 5 wt%loss.b The maximum weight loss rate temperature for the PPC.
Anchoring the organometallic species on the solid support has been achieved by a variety of routes involving the function‐al group of the support.Due to the air sensitivity of Zn Et2,the immobilization of the active center on the inorganic support was performed in propylene oxide reactant during the progress of the mixture of the reaction system under an inert atmos‐phere.Zn Et2 can react with hydroxyl groups on the silica sur‐face,which has been confirmed to be easily anchored[20],and a–Zn‐O‐Zn‐Et structure might be formed as theγ‐Al2O3‐sup‐ported analog[21].Table 1 shows the results of CO2/PO co‐polymerization over the Zn O/Si O2‐supported rare‐earth‐metal ternary catalyst.The activities for the Zn O/Si O2‐supported catalysts(Table 1,entries 3–5)increased compared to the sili‐ca‐supported one(Table 1,entry 2).The yield was at the level of 4009.2 g/mol Zn(based on the amount of Zn Et2 involved)with 3.0 g of 3 wt%Zn O/Si O2 support.As a typical amphoteric oxide,the Zn O‐modified silica catalyst showed higher activity than that of the acidic Al2O3‐modified one[12].Better disper‐sion of the active sites on Zn O/Si O2 might be obtained.Theadsorption of CO2 on Zn O[22]would also benefit the ring‐opening of PO and promote increased activity,though the Gibbs free energy of the adsorption and the insertion of CO2 into the Zn Et2 active site are relatively low,according to our calculation[12].
| 图表编号 | XD008192100 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.08.01 |
| 作者 | 程瑞华、周宇杰、侯侨丽、刘柏平 |
| 绘制单位 | 华东理工大学化学工程国家重点实验室、华东理工大学化学工程国家重点实验室、华东理工大学化学工程国家重点实验室、华东理工大学化学工程国家重点实验室 |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 2Thermal properties of the copolymer produced over rare‐earth‐metal ternary catalyst with various Zn O/Si O2 loadi”的人还看了
-
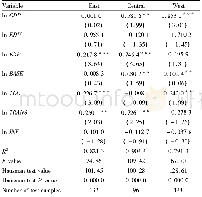
- Table 6 Regression analysis of factors affecting the development of agri-cultural producer service industry in the easte