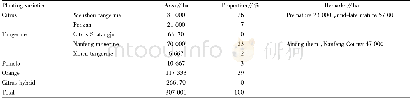
《Table 3.Products distribution of the n-octane hydroisomerization over the catalysts at the similar
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Effect of preparation method on the bimetallic NiCu/SAPO-11 catalysts for the hydroisomerization of n-octane》
A detailed product distribution for the catalysts at the similar conversion is shown in Table 3.For all the catalysts,the main n-octane isomers were 2-methyl heptane(2-MC7)and 3-methyl heptane(3-MC7).The ring opening of the octane protonated cyclopropane(PCP)intermediate at terminal position facilitated the formation of 2-MC7and 3-MC7[35].Among the di-methyl hexanes(DMC6)products,the 2,5-DMC6and 2,4-DMC6were major products,which was due to the further skeleton isomerization of2-MC7[35].The NiCu-CI,NiCu-CP,and CuNi-SI catalysts showed higher selectivity to n-octane isomers than the other catalysts.The NiCu-OMM catalyst showed the lowest selectivity to n-octane isomers.The Ni Cu-SI and Ni Cu-AM catalysts showed slightly higher selectivity to DMC6,but they also showed higher selectivity toβ-scission products.The cracking products containedβ-scission products and hydrogenolysis products.For the NiCu-OMM catalyst,it exhibited higher selectivity to cracking products than the other catalysts.The selectivity toβ-scission products for the Ni Cu-OMM catalyst was slightly higher than those for the other catalysts.However,the NiCu-OMM catalyst showed much higher selectivity to hydrogenolysis products than the other catalysts.Because the NiCu-OMM catalyst hardly formed the Ni-Cu alloy,the hydrogenolysis reaction was not restrained.For the cracking products over the Ni Cu-SI and Ni Cu-AM catalysts,the selectivity to hydrogenolysis products was low,indicating that the hydrogenolysis reaction was restrained.The selectivity to cracking products was due to the formation ofβ-scission products.Theβ-scission products were formed from the octane intermediates when the metal and acid sites were not balanced.Combined with the results shown in Figs.8 and 9,the NiCu-CI catalyst exhibited both high conversion of noctane and selectivity to n-octane isomers.Therefore,the Ni Cu-CI catalyst showed both excellent catalytic activity and isomerization selectivity in the hydroisomerization of n-octane.
| 图表编号 | XD0042894700 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.01.01 |
| 作者 | Zhichao Yang、Yunqi Liu、Yanpeng Li、Lingyou Zeng、Zhi Liu、Xueying Liu、Chenguang Liu |
| 绘制单位 | State Key Laboratory of Heavy Oil Processing, Key Laboratory of Catalysis, China National Petroleum Corp. (CNPC), China University of Petroleum (East China)、State Key Laboratory of Heavy Oil Processing, Key Laboratory of Catalysis, China National Petroleu |
| 更多格式 | 高清、无水印(增值服务) |