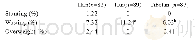《Table 3Herbal substances evaluated by the HMPC resulting in a Public Statement[9].》
The key steps mentioned may be helpful for planning and consideration during the very early stages of product development.In order to achieve progress and proceed to the development of a dossier,the project should be supported by competent partners in Europe(e.g.,partners in industry or consultants).The most valuable and essential recommendation is to consider scientific advice either by national authorities or at the level of the EMA(HMPC or CHMP).Scientific advice is available at any step of product development,and earlier is better for the applicant.The fees are reasonable,questions can address particular procedural or scientific issues,and the results are documented through a confidential protocol.Scientific advice may also be useful to elaborate major challenges in an early stage in order to allow wise decisions to be made about investments related to a project.In appropriate settings,scientific advice will substantially increase the chance of being successful.
| 图表编号 | XD0061015700 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.02.01 |
| 作者 | Werner Knoess、Jacqueline Wiesner |
| 绘制单位 | Federal Institute for Drugs and Medical Devices、Federal Institute for Drugs and Medical Devices |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 3Herbal substances evaluated by the HMPC resulting in a Public Statement[9].”的人还看了
-
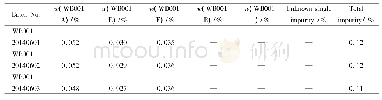
![Table 2Selected herbal substances evaluated by the HMPC resulting in a monograph or a list entry[9].](//bookimg.mtoou.info/tubiao/gif/GOCH201901006_09900.gif)
- Table 2Selected herbal substances evaluated by the HMPC resulting in a monograph or a list entry[9].
![《Table 3Herbal substances evaluated by the HMPC resulting in a Public Statement[9].》](http://bookimg.mtoou.info/tubiao/gif/GOCH201901006_10400.gif) 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
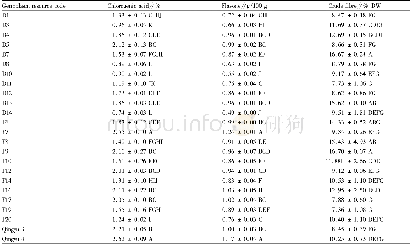
![Table 4Selected guidance documents published by EMA/HMPC[9].](http://bookimg.mtoou.info/tubiao/gif/GOCH201901006_10500.gif)
![Table 2Selected herbal substances evaluated by the HMPC resulting in a monograph or a list entry[9].](http://bookimg.mtoou.info/tubiao/gif/GOCH201901006_09900.gif)