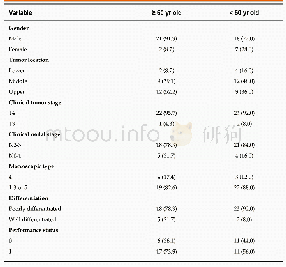
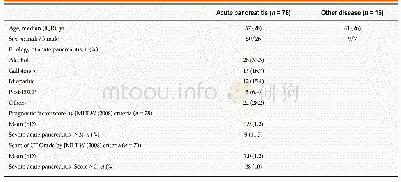
《Table 1Scientific committees at the EMA.》
An application must be submitted electronically and in a distinct format,as a Common Technical Document.The content depends on the type of procedure,as described above,and on the type of application:(1)Marketing authorization:full application—for new medicinal products,bibliographic application—for known medicinal products with well-established use,and hybrid forms may be used;(2)Registration:Bibliographic application with additional data on safety if necessary—only for traditional herbal medicinal products.
| 图表编号 | XD0061015200 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.02.01 |
| 作者 | Werner Knoess、Jacqueline Wiesner |
| 绘制单位 | Federal Institute for Drugs and Medical Devices、Federal Institute for Drugs and Medical Devices |
| 更多格式 | 高清、无水印(增值服务) |
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式