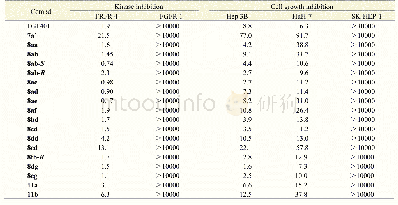
《Table 1 Cancer cellular immunotherapies approved by the Ethics Committee in China》
PSCA,prostate stem cell antigen;MUC1,mucin 1;PD-L1,programmed cell death ligand 1;EGFRvIII,epidermal growth factor receptor vIII;IL13Rα2,interleukin 13 receptorα2;EphA2,EPH receptor A2;GD2,ganglioside D2;GPC3,glypican-3;EpCAM,epithelial cell adh
In 1999,the China FDA issued“Guidelines for the Clinical Trial of Human Gene Therapy,”which regulates the relevant content of somatic cell therapy.In 2003,China promoted the“Guidelines for Human Cell Therapy Research and Preparation Quality Control Technology”to establish standard operating procedures for application materials,somatic cell collection,culture and in vitro operation,quality inspection and pre-clinical trial safety,and efficacy evaluation of adoptive cell transfer treatments,such as lymphokineactivated killer and TIL cells,and to provide a reference standard for somatic treatment in China.In 2013,based on the current development of ethics in China and documents,such as“Human Somatic Cell and Gene Therapy Conditions,”formulated by the US FDA Center in 1991 for biological products evaluation and research,the FDA of China issued the“Quality Control Points for Clinical Research of Human Somatic Cell Therapy and Gene Therapy,”which defined the basis of the research,identification of therapeutic cell populations,and safety and effectiveness of the evaluation of preclinical trials.It served as a foundation for examining the ethical issues related to cancer cellular immunotherapy in China.In December 2017,the“Guidelines for the Research and Evaluation of Cellular Therapeutic Products(Trial)”issued by the FDA of China provided guidelines for the development of clinical application rules for cancer cellular immunotherapy.At present,a number of clinical trials related to cellular immunotherapy,including those for lung cancer,stomach cancer,colorectal cancer,and liver cancer,have been reviewed and approved by the Chinese Ethics Committee(Table 1).
| 图表编号 | XD0047004400 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.01.03 |
| 作者 | Sang-sang REN、Jing-wen DENG、Meng HONG、Yan-li REN、Hai-jing FU、Yan-ning LIU、Zhi CHEN |
| 绘制单位 | Health Policy and Hospital Management Research Center, Department of Public Health, Zhejiang University School of Medicine、State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, Collaborative Innovation Center for Diagnosis and Treatment |
| 更多格式 | 高清、无水印(增值服务) |
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式