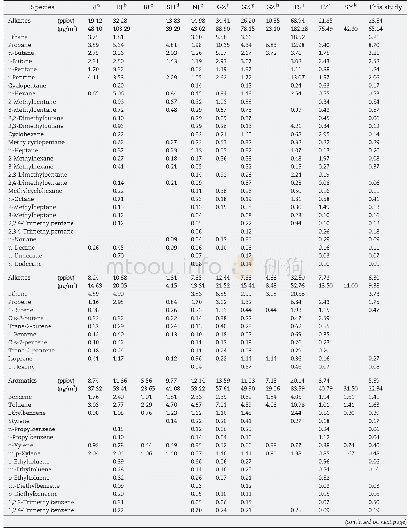
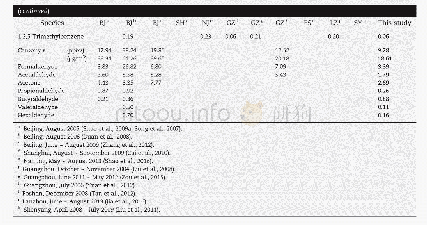
《Table 4–Comparison of carboxymethyl S-IIPs for this study with other adsorbents.》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《"Carboxymethyl cellulose thiol-imprinted polymers:Synthesis, characterization and selective Hg(Ⅱ) adsorption"》
where,Kdis the equilibrium constant obtained from the proportion of adsorbate on the polymer(qe)to that of the adsorbate in solution(Ce)at equilibrium.The slope and intercept of linear plot of Eq.(21)(Fig.10) gives values ofΔH°andΔS°respectively and results are shown in Table 5.Basically,ΔG°becomes more negative as temperature increases,thus demonstrating a more spontaneous reaction.TheΔS°(388.97 J/ (mol·K)) value was found to be positive,showing that randomness is heightened during the adsorption process.The calculated enthalpy of sorption value was positive indicating the endothermic nature of the Hg(II)adsorption process.UsuallyΔH°values that are less than 80 k J/mol point to the physical nature of the adsorption process(Parashar et al.,2016),otherwise chemisorption occurs.ΔH°was found to be greater than 80 k J/mol and this further confirms the chemisorption nature of the adsorption process,which is in agreement with the conclusion drawn from the calculated activation energy value of 51.54 k J/mol(Section 2.3.3).
| 图表编号 | XD0052062100 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.05.15 |
| 作者 | Tarisai Velempini、Kriveshini Pillay、Xavier Y.MbiANDa、Omotayo A.Arotiba |
| 绘制单位 | Department of Applied Chemistry, University of Johannesburg, Doornfontein Campus、Department of Applied Chemistry, University of Johannesburg, Doornfontein Campus、Centre for Nanomaterials, University of Johannesburg, Doornfontein Campus、Department of Scien |
| 更多格式 | 高清、无水印(增值服务) |