《Tab.2Kinetic and thermodynamic parameters of thermal decomposition for pyrotechnic mixtures》
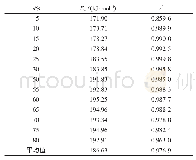
For the mixture of KMn O4+RP+Teflon,as seen in Tab.3,critical ignition temperature is 242.6℃,which is less than the critical ignition temperature of RP itself,indicating that the mixture of KMn O4+RP+Teflon was more reactive than RP itself.On the other hand,by replacing KMn O4with Mn O2and KNO3powder,the thermal sensitivity of the mixture decreases and ignition temperature of Mn O2+RP+Teflon and KNO3+RP+Teflon systems reach to 353.0℃and378.4℃.Finally,the replacement of KMn O4with Fe2O3and Ba(NO3)2raises the thermal stability of the mixture and ignition shifts toward the higher temperature up to 406.1℃and 421.1℃.Ba(NO3)2+RP+Teflon mixture with ignition temperature about 178℃higher than that most reactive mixture has the highest stability among the investigated mixtures.
| 图表编号 | XD004298900 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.08.20 |
| 作者 | 单聪明、刘杰、朱顺官、张琳 |
| 绘制单位 | 南京理工大学化工学院、南京理工大学化工学院、南京理工大学化工学院、南京理工大学化工学院 |
| 更多格式 | 高清、无水印(增值服务) |
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式