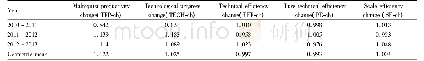
《Table 1.Mean theoretical and actual compositions in glasses》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Yb~(3+)/Al~(3+)/Ce~(3+)/F~–掺杂石英玻璃的结构与性质(英文)》
x is molar fraction;w is mass fraction.
Tetraethoxysilane(TEOS),C2H5OH,AlCl3·6H2O,YbCl3·6H2O,Ce Cl3·7H2O and(NH4)2Si F6 were used as precursors.Ethanol was utilized as a solvent.Deionized water was added to sustain the hydrolysis reaction.All the chemical reagents were analytically of pure grade.The preparation process of Yb3+-doped silica glass using the sol-gel method has been previously described[4,10].The sol was heated in an oxygen atmosphere from70 to 1 100°C to decompose the hydroxyl and organics.Then,the sol powder was sintered into glass at 1 750°C for 3 h in a vacuum state.The mean compositions of the glass samples are listed in Table 1.Si O2 was introduced with TEOS and(NH4)2SiF6;F was introduced by(NH4)2Si F6.Five samples with mean F/Si mass ratio of0%,2%,4%,8%and 12%were prepared and named YACF0,YACF2,YACF4,YACF8,and YACF12,respectively.Al3+single-doped(named Al)and Al3+/Yb3+co-doped(named Al/Yb)samples were prepared using the sol–gel method.Inductively coupled plasma atomic emission spectroscopy(ICP-AES)indicated that the contents of Yb2O3,Al2O3,and Ce2O3 in these silica glasses were close to the theoretical values as shown in Table 1.The F content was determined by electron probe microanalysis(EPMA,model JXA8230;JEOL Ltd.,Tokyo,Japan),with the test error being less than10%.A Heraeus Quarzglas quartz tube with a F-doping concentration of 65 000×10–6(mass fraction)was used as a reference as well.The actual content of F was remarkably lower than the corresponding theoretical value due to F volatilization during high-temperature sintering process(Table 1).
| 图表编号 | XD0032556600 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.01.01 |
| 作者 | 邵冲云、王璠、郭梦婷、杨佳慧、张海波、李吉豪、王世凯、于春雷、任进军、胡丽丽 |
| 绘制单位 | 中国科学院上海光学精密机械研究所、中国科学院大学、中国科学院上海光学精密机械研究所、中国科学院大学、中国科学院上海光学精密机械研究所、中国科学院大学、布鲁克(北京)科技有限公司、中国科学院上海光学精密机械研究所、中国科学院上海应用物理研究所、中国科学院上海光学精密机械研究所、中国科学院上海光学精密机械研究所、中国科学院上海光学精密机械研究所、中国科学院上海光学精密机械研究所 |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 1.Mean theoretical and actual compositions in glasses”的人还看了
-

- Table 6 Use efficiency of fertilizers to the actual yield of rice in the early and late rice cropping fields and LAD