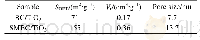《Table 2.Microwave absorption properties of different solutions》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Recovery of vanadium and molybdenum from spent petrochemical catalyst by microwave-assisted leaching》
Fig.9 exhibits the effect of agitation on the solution temperature in the microwave field.The heating rate of the solution in the stagnant state is lower than that in the agitated state.Given the location of the temperature sensor,the actual solution temperature should be higher than the measured value because of existence of a thermal gradient in solution.Thermal transmission can be promoted under agitation,and the variation of measured temperature in the agitated state is higher than that in the stagnant state at the same microwave power.This finding indicates the existence of a microwave penetration depth(MPD)in the solution under microwave radiation and the absence of positive“entire uniform heating”of microwave technology.On account of the presence of limited MPD,the solution in the microwave penetration district would heat up rapidly and the temperature of the entire reaction system could increase on the basis of heat transmission,which indicates the existence of a thermal gradient between reactor wall and central area.The coaxial probe method(DAK-3.5 SPEAG)was adopted to measure the microwave absorption property of the solution.A summary of the microwave absorption properties of different solutions is provided in Table 2.The MPD of solutions with reagents(ferric sulfate,sulfuric acid,hydrogen peroxide,and Na OH)are shallower than that of distilled water.This result is due to the influence of reagents on the microwave absorption properties of the solution.The dielectric constant and loss coefficient of NaOH solution(2.0mol·L-1)at 90°C and 2450 MHz are 27 and 55,respectively.The MPD calculated using Eq.(1)is 2.36 mm[27],which means that the large mass of microwave energy is gradually weakened within 2.36 mm of the reactor.Under the stagnant condition,most of the spent catalysts are located at the bottom of the reaction vessel.Moreover,only a handful of particles are within the high-efficiency microwave working area,indicating that few particles will be heated efficiently under microwave irradiation.Under the agitated condition,the particles would be thrown into the shallow outside layer of the reaction vessel,which is located in the MPD area[27].
| 图表编号 | XD0028408000 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.01.01 |
| 作者 | Zhi-yuan Ma、Yong Liu、Ji-kui Zhou、Mu-dan Liu、Zhen-zhen Liu |
| 绘制单位 | Guangdong Institute of Resources Comprehensive Utilization, Guangdong Academy of Sciences、State Key Laboratory of Separation and Comprehensive Utilization of Rare Metals、Guangdong Provincial Key Laboratory of Development and Comprehensive Utilization of M |
| 更多格式 | 高清、无水印(增值服务) |