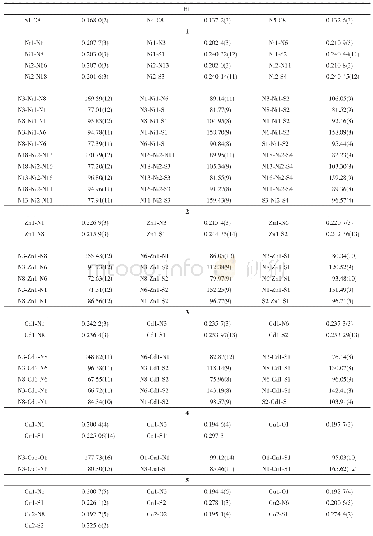
《Table S2 Selected bond lengths[]and angles[°]for 1 at 208,300 K and 323 K》
![《Table S2 Selected bond lengths[]and angles[°]for 1 at 208,300 K and 323 K》](http://bookimg.mtoou.info/tubiao/gif/SCMA202011012_15300.gif) 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《全温度覆盖的三重介电及荧光响应无铅杂化钙钛矿开关材料(英文)》
Other phases were compared with the specific crystal data(Table S1),packing structures (Figs 3 and 4),and molecular structures (Fig.5)to discuss their differences and similarities.At 208 K of Phase III,it crystallized in space group Pmcn,which also belongs to the orthorhombic crystal system with a=9.6079?,b=14.2701?,and c=6.4163?.As shown in Fig.3a,linear[Mn Cl3]nchains(along the c axis)of face-sharing Mn Cl6octahedra construct the framework of the crystal lattice,of which the Mn atoms and one-third of the Cl atoms lie in the mirror plane,while the other Cl atoms do not.This mirror also passes through the oxygen atoms of TMNO cations,as shown in the sky blue area of Fig.3a.When the temperature rises to 300 K of Phase II,all the Mn–Cl bond lengths and Cl–Mn–Cl bond angles are changed within the normal ranges(Table S2).It is worth noting that the dynamics of their organic cations change from relatively stationary motions to tumbling motions,causing the phase transition.As shown in Fig.3b,all Cl atoms are in the mirror plane,while Cd atoms are not.The tumbling motions of TMNO cations are symmetrical in the mirror plane,and all C atoms lie in the mirror plane too.These differences can be attributed to the change of symmetry elements.In general,the overall unit cell frameworks at 208 and 300 K have almost no differences(Fig.3).The main reason for the phase transition (Phase III to Phase II)is the movement of organic cations(Fig.5a,b).
| 图表编号 | XD00224915100 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2020.11.01 |
| 作者 | 张铁、储璐璐、张志旭、李杰、张婉莹、石萍萍、叶琼、付大伟 |
| 绘制单位 | Ordered Matter Science Research Center, Jiangsu Key Laboratory for Science and Applications of Molecular Ferroelectrics, Southeast University、Institute for Science and Applications of Molecular Ferroelectrics, Key Laboratory of the Ministry of Education f |
| 更多格式 | 高清、无水印(增值服务) |
![Table S2 Selected bond lengths[]and angles[°]for 1 at 208,300 K and 323 K](http://bookimg.mtoou.info/tubiao/gif/SCMA202011012_15301.gif)