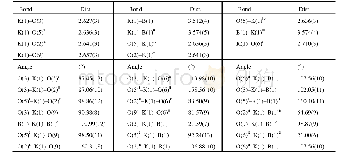
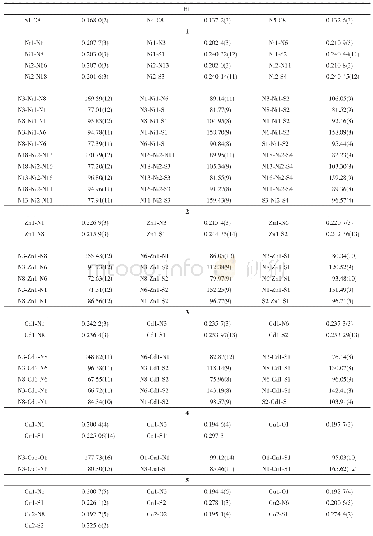
《Table 1.Selected Bond Lengths () and Bond Angles (°) for Cu LCl2》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Antifungal Evaluation of Copper(Ⅱ) Complex from a Schiff Base Derivative of Pyrazine on Schizosaccharomyces pombe》
Diffraction intensities for the complex were collected on a CrysAlisPro Agilent Technologies diffractometer with mirror-monochromator Cu Kαradiation(λ=1.5418?).Empirical absorption correction using spherical harmonics,implemented in SCALE3 ABSPACK scaling algorithm.The structures were solved by direct methods and refined with full-matrix least-squares technique using SHELXS-97 and SHELXL-97 programs,respectively.Anisotropic thermal parameters were applied to all non-hydrogen atoms.The organic hydrogen atoms were generated geometrically(methyl C-H0.96?,methylene C-H 0.97?,pyrazinyl C-H0.93?,and N-H 0.90?).Complex CuLCl2 crystallizes in triclinic,space group P 1with a=6.9064(5),b=8.8060(6),c=10.1255(6)?,?=69.913(6)o,β=73.529(6)o,?=83.682(6)o,V=554.56(6)?3,Z=2,Cu(C8H12N4)Cl2,Mr=298.66,Dc=1.789 g/cm3,μ=6.985 mm-1,S=1.075,F(000)=302,the final R=0.0364 and wR=0.0998 for1811 observed reflections(I>2σ (I)) .Rint=0.0281(1931 independent reflections),(Δρ) max=0.522 and(Δρ)min=–0.961 e/?3.Selected bond distances and bond angles are listed in Table 1.
| 图表编号 | XD0034854400 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.01.01 |
| 作者 | 杨平 |
| 绘制单位 | School of Environment, Jinan University |
| 更多格式 | 高清、无水印(增值服务) |