《Table 1 A summary on the noncovalent interactions present in peptide-based hydrogels》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《The Physical Chemistry for the Self-assembly of Peptide Hydrogels》
Because short-peptide hydrogels comprise of selfassembled fibrous networks,the maximum stretching force,which the network can tolerate,is defined by the non-covalent interactions among individual peptide sequences as well as the physical crosslinks among different fibrils.These interactions are generally weak compared to covalent interactions.Therefore,peptide hydrogels are soft and prone to erosion.Stabilizing intermolecular interactions is essential for the formation of short-peptide hydrogels.The weak interactions that favor intermolecular binding include hydrophobic effects[41,42],ionic interactions[43],hydrogen binding[44],π-πstacking[45-47],cation-πinteractions[48]and intrinsic sequence propensity(Table 1).Generally,hydrophobic effects can be understood as the effects to minimize the entropic cost for inserting a hydrophobic substance into dynamic hydrogen bonding network of water molecules[49].Hydrophobic effect is the major driving force for protein folding and the self-assembly of peptide hydrogelators.However,hydrophobic effects alone cannot provide directionality for self-assembly.With only hydrophobic effects as the driving force,peptides favor the formation of micelles instead of nanofibrils[50].Ionic interactions between opposite charges can be very strong in vacuum with a bonding energy of~100 kBT[51].However,it is much reduced due to the formation of hydrated ions in water and the charge screening effects from salts.Ionic interactions alone also do not provide directionality for self-assembly.On the contrary,hydrogen bonding is directional and provides strong stabilizing effect for protein secondary structures and peptide fibrils.Hydrogen bonds can be formed among the amino and carboxyl groups in backbone and side chains.Hydrogen bonds are very sensitive to the distance and direction between hydrogen donor and acceptor pairs[52]and thus play the pivotal role for the formation of one-dimensional(1D)nanofibrils of peptide hydrogelators[53].The strength of hydrogen bonds is in a range of 5-10 kBT at room temperature.π-πStacking and cation-πinteractions originate from the quadrupole-quadrupole interactions betweendelocalizedelectronsinp-orbitalsor quadrupole-monopole interactions between delocalized electrons in p-orbitals and adjacent cations[48,54].They can also provide directionality for self-assembly despite the fact that the effect is limited to a few aromatic amino acids.
| 图表编号 | XD0020168000 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.03.01 |
| 作者 | Ying Li、Yi Cao |
| 绘制单位 | Jiangsu Joint Laboratory of Atmospheric Pollution Control, Nanjing University of Information Science & Technology、Collaborative Innovation Center of Advanced Microstructures, National Laboratory of Solid State Microstructure, Department of Physics, Nanjin |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 1 A summary on the noncovalent interactions present in peptide-based hydrogels”的人还看了
-
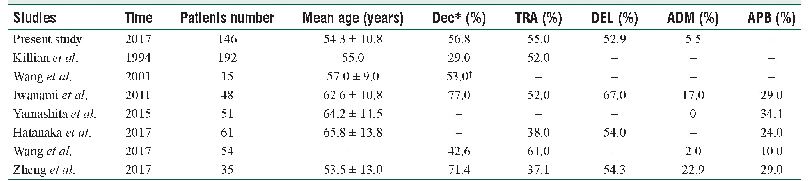
- Table 3:Comparison of previous reports and the present study on frequency and distribution of decremental responses in p
-
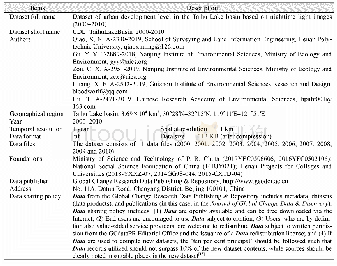
- Table 1 Metadata summary of“Dataset of urban development level in the Taihu Lake basin based on nighttime light images(2
-
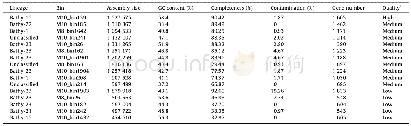
- Table 1General characteristics of the MAGs retrieved in the present study from Guaymas Basin hydrothermal sediments.