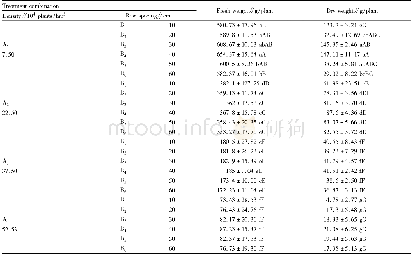
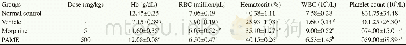《Table 6 Influences of the pilot product of QFJBT on hematological parameters of rats in a 3-month,
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《采用“化学、药效与毒理”三结合方法筛选优化抗关节炎中药复方青附蠲痹汤的最佳制备工艺(英文)》
Notes:data are presented as the mean±SD(n=11~12).Abbreviation:RBC represents erythrocyte count;WBC represents white blood cell count;HGB represents hemoglobin concentration;HCT represents hematocrit;LYM represents lymphocyte count;LY represents total a
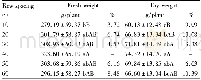
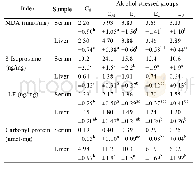
In the chronic oral toxicity studies,about 67%animals treated with QFJBT at the dose of 9.72 g/kg(equivalent to 100-fold clinical dosage)showed mild or moderate decrease of activity and diarrhea in the initial stage of the experiment(7~14 d after the initiation of QFJBT treatment).The results of laboratory investigation showed that all hematological parameters(erythrocyte,leukocyte and platelet counts,hemoglobin concentration,hematocrit,lymphocyte and differential lymphocyte counts)(Table 6) and bloodbiochemicalparameters(aspartate aminotransferase,alanine aminotransferase,alkaline phosphatase,total protein,albumin,total bilirubin,glucose,creatinine,triglycerides)(Table 7) of QFJBT-treated rats fell within the range of normal biological variation as compared with vehicle-treated rats.No mortality or specific toxic responses or treatment-induced pathological changes in all test organs and tissues were observed in animals after 3months of repeated dosing with QFJBT.
| 图表编号 | XD0014720700 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.12.01 |
| 作者 | 张婷、余黄合、林也、李鑫、唐琳、宋厚盼、彭清华、王炜、刘良、陈聪、蔡雄 |
| 绘制单位 | 湖南中医药大学中药粉体与创新药物省部共建国家重点实验室(培育基地)、湖南中医药大学中医学院、湖南中医药大学中药粉体与创新药物省部共建国家重点实验室(培育基地)、湖南中医药大学药学院、湖南中医药大学中药粉体与创新药物省部共建国家重点实验室(培育基地)、湖南中医药大学中医学院、湖南中医药大学中医学院、广东药科大学中药学院、湖南中医药大学中医学院、湖南中医药大学中医学院、澳门科技大学中药质量研究国家重点实验室、湖南中医药大学中药粉体与创新药物省部共建国家重点实验室(培育基地)、湖南中医药大学中医学院、湖南中医药 |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 6 Influences of the pilot product of QFJBT on hematological parameters of rats in a 3-month, repeat-dose oral toxi”的人还看了
-
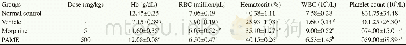
- Table 1Influence of PAME on hematological parameters after vincristine induced neuropathic pain in rats.