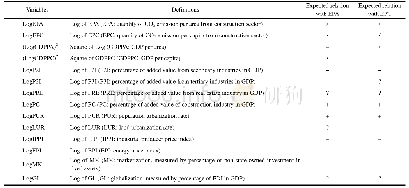
《Table 1 Structural and thermal data related with microphase separation of BBCP, and parameters rela
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Random Binary Brush Architecture Enhances both Ionic Conductivity and Mechanical Strength at Room Temperature》
a Lithium-doping ratio;b Tg of BBCP by DSC;c Calculated from fitting the conductivity with VFT equationσDC=σ0exp{-B/(T-T0)};d q*is the scattering vector at the maximum scatting intensity from SR-SAXS curves.
The binary brush structure has positive effect on the ionic conductivity.Figure 6 compares the temperature dependence of DC conductivityσDC of the samples obtained from the DRS measurements,and that reported by Floudas and coworkers for the PEO/Li Tf mixtures with Mn=14000[41].TheσDC exhibits Vogel-Fulcher-Tammann(VFT)-type temperature dependence in a wide temperature range.The parameters are summarized in Table 1.In general,the conductivity decreases with increasing lithium doping ratio.It is related with the molecular architecture and the phase segregated structure induced by lithium ion.From the point of the polymer chain movement,the mobility of the lithium ions is limited due to Tg increase with increased lithium doping ratio.The incomplete phase segregation induced by lithium ion doping results in boundaries and defects in the nanodomains,as well as out of control of the orientation of the nanodomains.For r=0.05,the liquid-like PEO domains in the weak microphase segregated structures facilitate ion conductivity,because the lithium ions migrate more easily between randomly dispersed,flexible PEO chains,compared to the well separated and stiff nanodomains.Another feature of the conductivity curves is that no abrupt transition of conductivity is seen as the function of temperature.For PEO/Li Tf mixtures,in contrary,abrupt drops of conductivity are observed across PEO melting point(1000/T>3.0)for all the lithium doping ratios.That is to say,the ionic conductivity at room temperature is low.We now focus on the r=0.05system,in which the crystallization of PEO(Mn=14000)is successfully suppressed due to random introduction of PS brushes into BBCP.Therefore,the ionic conductivity at temperature below PEO melting point is higher than the reported data.
| 图表编号 | XD0020167100 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.01.01 |
| 作者 | Yu-Feng Lyu、Zhi-Jie Zhang、Chang Liu、Zhi Geng、Long-Cheng Gao、Quan Chen |
| 绘制单位 | Key Laboratory of Bio-inspired Smart Interfacial Science and Technology of Ministry of Education, School of Chemistry and Environment, Beihang University、State Key Laboratory of Polymer Physics and Chemistry, Changchun Institute of Applied Chemistry, Chin |
| 更多格式 | 高清、无水印(增值服务) |