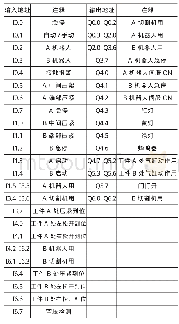
《Table 1 Patients allocation》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《定心方联合胺碘酮治疗室性早搏:一项随机、双盲、多中心、安慰剂对照试验(英文)》
This trial was conducted at 7 centers in China from July2012 to December 2013(Table 1).The primary objective was to assess the efficacy of DXR combined with amiodarone for the treating of PVCs.The secondary objective was to assess the safety and tolerability of DXR combined with amiodarone in this relevant patient population.The study protocol,approved by the institutional review board of each institution,was conducted in accordance with the Declaration of Helsinki.Informed consent was obtained from all patients before entering the trial.Patients were free to withdraw from the trial at any time.An independent steering committee of academic physicians was responsible for the design and conduct of the trial,data analysis,and reporting of the trial.A data monitoring committee oversaw the safety of patients.This trial is registered with the Chinese Clinical Trial Registry.The protocol identification number at http://www.chictr.org.cn is ChiCTR-TRC-12002794.
| 图表编号 | XD0014716200 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.03.01 |
| 作者 | 周凤华、贾钰华、叶遂林、娄林洁、杨平、李杰、张月均、钱荣江 |
| 绘制单位 | 南方医科大学、南方医科大学、广州中医药大学、温州大学第一附属医院、江西中医药大学附属中西医结合医院、山西省中医院、横店市人民医院、宁海县中医院 |
| 更多格式 | 高清、无水印(增值服务) |