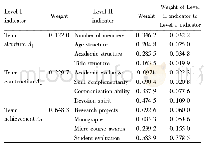
《Table 1 Course of EXCO development of Al-Cu-Li alloy joint》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《时效处理对铝锂合金电子束焊接头耐蚀性能的影响(英文)》
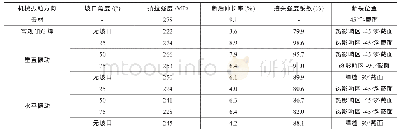
When electrochemical corrosion occurs in metal material,corrosion rate depends on the value of self-corrosion current(or self-corrosion current density).Usually,the higher its self-corrosion current is,the higher the corrosion rate is,and the corrosion dimension is deeper in a unit time;thus thecorrosion in the material is more severe,and it means a low corrosion resistance.The polarization curves of Al-Cu-Li alloy weldment before and after aging treatment in3.5%Na Cl solution are shown in Fig.5.The electrochemical corrosion parameters obtained by analyses of polarization curves are given in Table 2.From Fig.5 and Table 2,it can be seen that the self-corrosion potential decreases lightly after PWHT,which demonstrates that the possibility to corrosion increases.Compared to the self-corrosion current of weldment in AW condition,the self-corrosion current of weldment increases after PWHT,which leads to the increase of corrosion rate of weldment.
| 图表编号 | XD0011249900 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.07.01 |
| 作者 | 王少刚、黄燕、赵礼 |
| 绘制单位 | 南京航空航天大学、南京航空航天大学、南京航空航天大学 |
| 更多格式 | 高清、无水印(增值服务) |