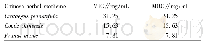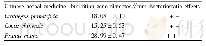《Table 2Cytotoxicity of compounds 1–12 against three human cancer cell lines.》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Chemical constituents from Kalanchoe hybrida and their cytotoxicity》
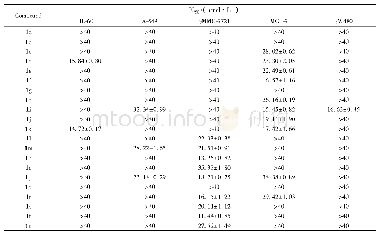
Among these isolates,compounds 1,2,and 4–12 were screened for their in vitro cytotoxicity against MCF-7,NCI-H460,and SF-268 tumor cell lines with previously reported assay(Cheng et al.,2003),and the data were given in Table 2.Compounds 1 and 2did not exhibit any significant cytotoxicity at 4μg/m L towards all the three tested tumor cell lines.But kalanhybrin A(1)displayed82%inhibition against NCI-H460 cell lines at 20μg/m L.Compounds4–8 displayed significant cytotoxicities towards all the tumor cell lines with the inhibition percentages higher than 50%at the tested concentrations.Among these,the principles of 5–8 were comparatively not effective towards the SF-268 tumor cell lines at the concentration of 4μg/m L.These experimental data indicated that theα-pyrone ring is necessary for the cytotoxicity while comparing 1–2 with 4–7.Moreover,theγ-lactone ring derivative 8 which displayed weaker cytotoxicity suggested that the ring size was also a factor to the bioactivity.In addition,the lignans 9 and 10,and the flavonoids 11 and 12,which were purified from the n-butanol fraction,were examined for their cytotoxicity and only kaempferol-3-O-α-D-(2-O-β-D-xylosyl)rhamnoside(11)exhibited significant inhibitions against the tested tumor cell lines.It suggested that 11should be an active principle in the n-butanol fraction of methanol extract of K.hybrida.
| 图表编号 | XD007997700 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.04.18 |
| 作者 | Ping-chung Kuo、Hsin-yi Hung、Yu-ren Liao、Yu-yi Chan、Tian-shung Wu |
| 绘制单位 | School of Pharmacy, College of Medicine, National Cheng Kung University、School of Pharmacy, College of Medicine, National Cheng Kung University、School of Pharmacy, College of Medicine, National Cheng Kung University、Department of Biotechnology, Southern T |
| 更多格式 | 高清、无水印(增值服务) |