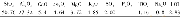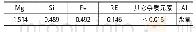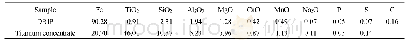《Table 2 Chemical compositions of the calcined samples》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Preparation of CeO_2-Modified Mg(Al)O-Supported Pt–Cu Alloy Catalysts Derived from Hydrotalcite-Like Precursors and Their Catalytic Behavior for Direct Dehydrogenation of Propane》
a The numbers in parentheses are the nominal compositions in the synthesis mixture and those without parentheses are determined by ICP-OESanalysis
The calculated lattice parameters of the precursors are listed in Table 1.The a values of CuMgAl-P,Ce 3.0 MgAl-P,and PtCuCe x MgAl-P samples increase compared to that of MgAl-P.Additionally,the presence of small amounts of Ce 3+ions gives rise to an increasing value of lattice parameter a across PtCuCe 0.1 MgAl-P,PtCuCe 0.3 MgAl-P,and PtCuCe 0.6 MgAl-P,followed by a decrease with further increase in the Ce content.For CuMgAl-P,the octahedron becomes larger because of the decrease of positive charge density in the layers incorporated with Cu 2+ions.As for the Ce-containing samples,it is mainly because Ce 3+ions have a bigger radius than Al 3+ions,which can broaden the distance between metal cations in the layers and increase the a value[31].The corresponding lattice parameter c exhibits a similar trend.Although the reasons are diff erent,it can be confi rmed that Cu 2+and Ce 3+ions can be introduced in the HT structure layers as the amounts of Cu 2+and Ce 3+ions are small.
| 图表编号 | XD0076872800 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.04.01 |
| 作者 | Yingxia Li、Jiaxin Li、Xiao Yang、Xitao Wang、Yanhong Xu、Lihong Zhang |
| 绘制单位 | School of Chemical Engineering and Technology, Tianjin University、Tianjin Key Laboratory of Applied Catalysis Science and Technology, Tianjin University、School of Chemical Engineering and Technology, Tianjin University、Tianjin Key Laboratory of Applied Ca |
| 更多格式 | 高清、无水印(增值服务) |