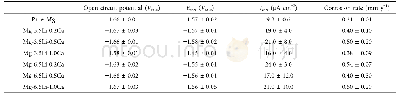
《Table 5 Corrosion potential (φcorr) and corrosion current density (Jcorr) of samples A, B, C and D
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《具有均匀和双峰晶粒尺寸分布的电沉积镍的腐蚀行为(英文)》
Figure 13 shows the potentiodynamic polarization curves for samples A,B,C and D in 0.5 wt.%H2SO4solution,withthecorrespondingelectrochemical parameters listed in Table 5.The corrosion behavior is quite different from that in NaOH or NaCl solution as there is no passivation phenomenon.Theφcorr shifts positively from-0.39 to-0.15 V,whilst the Jcorr decreases from 31.18 to 15.80μA/cm2 with increasing the grain size in the sample.It is clear that the smaller the grain size is in the sample,the more the grain boundaries are and the lower the corrosion resistance is,withou passivation.This will increase the electrochemica reactivity and accelerate the corrosion of Ni[34].
| 图表编号 | XD0064475900 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.02.01 |
| 作者 | 杨峰、张晓峰、杨虹、刘亦农、郝世杰、崔立山 |
| 绘制单位 | 中国石油大学(北京)重质油国家重点实验室、中国石油大学(北京)油气装备材料失效与腐蚀防护北京市重点实验室、中国石油大学(北京)重质油国家重点实验室、中国石油大学(北京)油气装备材料失效与腐蚀防护北京市重点实验室、School of Mechanical and Chemical Engineering,The University of Western Australia、School of Mechanical and Chemical Engineering,The University of Wes |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 5 Corrosion potential (φcorr) and corrosion current density (Jcorr) of samples A, B, C and D measured in 0.5 wt.%H”的人还看了
-

- 表2 Ni-Si C复合镀层的腐蚀电位Ecorr和腐蚀电流密度Icorr Table 2 Corrosion potential Ecorrand corrosion current density Icorrof Ni-Si C comp
-

- Table 3 Corrosion potential (φcorr) and corrosion current density (Jcorr) of samples A, B, C and D measured in 3 wt.%Na
-

- Table 1 Corrosion potential (φcorr) , corrosion current density (Jcorr) , passive current density (Jpass) and passive ra
-

- 表2 实验钢的自腐蚀电位和腐蚀电流密度Tab.2 Self corrosion potential and corrosion current densities of experimental steels