《Table 2 Corrosion potentials (Ecorr) and corrosion current densities (icorr) of Mg-Al-Pb (A) and Mg
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Mg-Al-Pb及Mg-Al-Pb-Zn合金在3.5% NaCl溶液中的腐蚀行为(英文)》
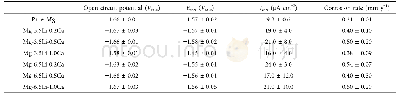
Fig.3 shows the potentiodynamic polarization curves of A and B alloys in different heat treatment conditions immersed in 3.5%NaCl aqueous solution at 25℃.Each measurement started immediately after the specimen was immersed in NaCl aqueous solution.The polarization curves are not symmetrical in the anodic and cathodic branches.The current density increases in the anodic polarization branch is much greater than that in the cathodic branch.At more negative potentials than the corrosion potential(Ecorr),evolution of hydrogen dominates and results in cathodic current.At more positive potentials than Ecorr,magnesium oxidation dominates and the metal is continuously dissolved with the help of Cl-ions,which can make the oxide film break down.The corrosion current densities(icorr)and the corrosion potentials(Ecorr)of all these specimens are listed in Table 2.The corrosion current density can be ranked in a decreasing order:A-T4>B as-cast>B-T4>A as-cast.The corrosion current density(icorr (mA/cm2)is related to the average penetration rate(Pj (mm/y)) using Eq(1)and the results are listed in Table 3.For an alloy,the solution treatment results in the decrease of the corrosion rate,because theβ-phase without zinc mainly serves as the corrosion barrier to inhibit the corrosion process.
| 图表编号 | XD0010568500 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.06.20 |
| 作者 | 殷立勇、周威、王平安 |
| 绘制单位 | 中国电子科技集团第十八研究所、中国电子科技集团第十八研究所、中国电子科技集团第十八研究所 |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 2 Corrosion potentials (Ecorr) and corrosion current densities (icorr) of Mg-Al-Pb (A) and Mg-Al-Pb-Zn (B) alloys”的人还看了
-

- 表2 Ni-Si C复合镀层的腐蚀电位Ecorr和腐蚀电流密度Icorr Table 2 Corrosion potential Ecorrand corrosion current density Icorrof Ni-Si C comp
-

- Table 5 Corrosion potential (φcorr) and corrosion current density (Jcorr) of samples A, B, C and D measured in 0.5 wt.%H
-

- Table 3 Corrosion potential (φcorr) and corrosion current density (Jcorr) of samples A, B, C and D measured in 3 wt.%Na
-

- Table 1 Corrosion potential (φcorr) , corrosion current density (Jcorr) , passive current density (Jpass) and passive ra