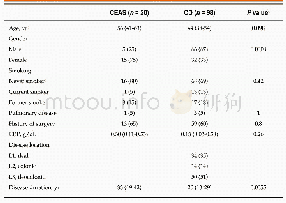
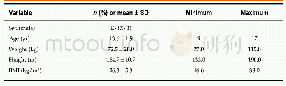
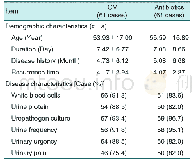
《Table 1.Clinical Characteristics of CKD Participants[Median (IQR) ]》
![《Table 1.Clinical Characteristics of CKD Participants[Median (IQR) ]》](http://bookimg.mtoou.info/tubiao/gif/ZXYY201903002_07300.gif) 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《"Effects of Niaoduqing Particles(尿毒清颗粒)on Delaying Progression of Renal Dysfunction:A Post-trial,Open-Label,Follow-up Study"》
Notes:IQR:interquartile range;eGFR:estimated glomerular filtration rate;Scr:serum creatinine;SBP:systolic blood pressure;DBP:diastolic blood pressure;UPE:urinary protein excretion
The patient enrollment protocol for the doubleblind trial period was published previously.(15)As shown in Figure 1,146 participants in each group entered the full analysis set(FAS).The characteristics of the entire trial cohort at baseline,post-doubleblind period and post-open-label period are shown according to the original study-group assignments,and there were no significant differences at the three periods between Niaoduqing and placebo groups(age:50.8±11.7 vs.50.7±11.9 years;male proportion:56.9%vs.56.2%,P>0.05,Table 1).
| 图表编号 | XD0064291500 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.03.01 |
| 作者 | ZHENG Ying、WANG Nian-song、LIU Yu-ning、HE Li-qun、JIAN Gui-hua、LIU Xu-sheng、NI Zhao-hui、CHENG Xiao-hong、LIN Hong-li、ZHOU Wen-hua、WANG Ya-ping、FANG Jing-ai、HE Ya-ni、YANG Hong-tao、ZHAO Li-juan、DING Han-lu、WANG Li-hua、YU Ren-huan、LI Wen-ge、YE Zhi-ming、GUO Wang |
| 绘制单位 | Department of Nephrology, Chinese People's Liberation Army General Hospital, Chinese People's Liberation Army Institute of Nephrology, State Key Laboratory of Kidney Diseases (2011DAV00088), National Clinical Research Center for Kidney Diseases、Departme |
| 更多格式 | 高清、无水印(增值服务) |