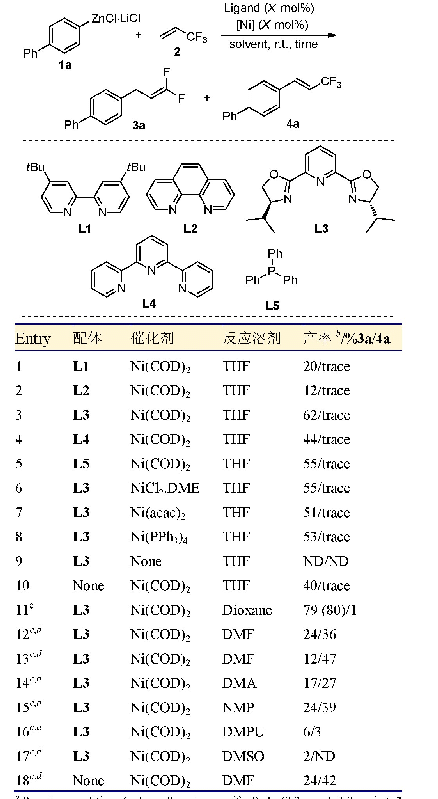
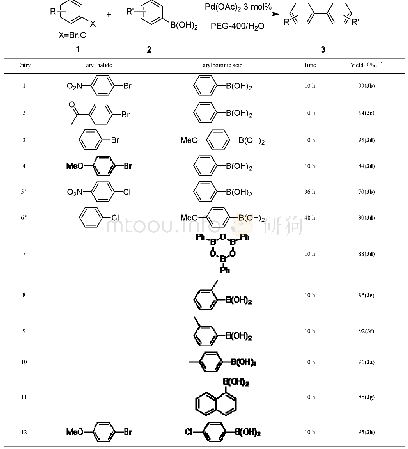
《表2 芳基硼酸与卤代苯的Suzuki偶联反应 (催化剂为1和2)》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《基于纳米二氧化硅与MCM-41的两种多相钯催化剂的合成及其在Suzuki反应中的应用(英文)》
(GC,Gas chromatograph yield.*Column yield)
In order to demonstrate the efficiency and the scope of the catalysts(1,2),several kinds of aryl halides and arylboronic acids were employed under the optimal reaction conditions to produce the corresponding biphenyls.As shown in Tab.2,under the same conditions,using catalyst 1 and 2 is almost the same for most reactions.When chlorobenzene was used,the yields are very low.Even if the reaction temperature is increased to 80℃,the yields of the reactions are still not high(Entry 2,3 and11,12).When the aryl boric acid contains halogen substituents(F,Cl),the yields are also significantly reduced(Entry 8,9 and 17,18).Furthermore,when brominated aryl and iodide aryl compounds react with benzene boric acid(Entry 4~7 and 13~16),the yields are usually good.
| 图表编号 | XD0052530000 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.04.18 |
| 作者 | 曹锰、王振希、张健、徐胜、张尚玺、柳阳、戴欣、江新德、吴锦辉 |
| 绘制单位 | 南昌工程学院理学院、南昌工程学院理学院、南昌工程学院理学院、南昌工程学院理学院、南昌工程学院理学院、南昌工程学院理学院、南昌工程学院理学院、南昌工程学院理学院、南昌工程学院理学院 |
| 更多格式 | 高清、无水印(增值服务) |



![表1 催化剂Pd[2Oxime-mim]BF4催化不同底物的Suzuki偶联反应](http://bookimg.mtoou.info/tubiao/gif/HXYZ202004004_03600.gif)