《Table 4.The TPR data for the calcined catalysts.》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Fischer–Tropsch synthesis using Co and Co-Ru bifunctional nanocatalyst supported on carbon nanotube prepared via chemical reduction method》
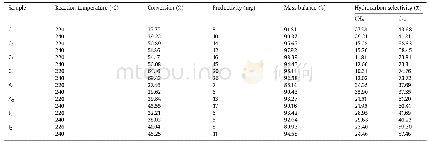
aDetermined from 423 to 723 K.
Fig.7 demonstrates the TPR profiles for Co and Co-Ru based catalysts so that the reduction behavior of the present catalysts showed two major reduction peaks as a low temperature peak(330-385°C)and the high temperature peak(400-500°C).The first peak typically corresponded to the reduction of Co3O4to CoO through the reaction Co3O4+H2→3CoO+H2O and the second peak was mainly ascribed to the second step of reduction from Co O to metallic cobalt by reaction Co O+H2→Co0+H2O that verify the XRD patterns.These observations were in agreement with those given in the previous works[3,10,16,18,21].Table 4shows the specific reduction temperatures corresponding to those demonstrated in Fig.7.Moreover,a small reduction peak about621.3°C was seen at the TPR spectrum of A1catalyst,which would be attributed to the reduction of the cobalt aluminate formation through strong interaction between cobalt and the alumina support so that the Co3O4reduction was diminished[7–8,28].However,this peak vanished for A2catalyst,i.e.Co-Ru/γ-Al2O3,which indicated no interaction between cobalt and alumina that was in agreement with the XRD results[7,18,45].As demonstrated in Fig.7,a decrease in the reduction temperatures for Co-Ru/CNTs in comparison to Co-Ru/γ-Al2O3catalysts indicated higher cobalt particles deposition at the CNTs[27,42]so that the degree of interaction between cobalt and CNTs was much weak in compared to the interaction between Co andγ-Al2O3.In the other words,based on the different studies[8,27–28,42],CNTs presented an easier reduction process in compared toγ-Al2O3,because the CNTs presented as an inert support that didn’t demonstrate any peak related to the formation of oxide compounds.Therefore,in the CNTs supported catalysts more cobalt atoms were available to participate in FT synthesis in comparison with the alumina-supported catalysts[8].Moreover,Fig.7 indicates that both TPR peaks significantly were shifted to the lower temperatures by loading Ru to the Co/CNTs catalyst(C1).On the other hand as shown in Table 4,by increasing the ratio of Ru/Co from zero to 0.4,the both reduction peaks were shifted from 356°C to 329.8°C and 460.4°C to 409.7°C.Therefore,the Co3O4species would be reduced easier to Co active sites at CNTs for FT synthesis,which led to higher reduction degree for the bimetallic catalysts of Co-Ru.On the other hand,owing to high hydrogen dissociation and activation of ruthenium,this led to decrease reduction temperature of Co particles by Hadsspillover from ruthenium to cobalt[7].Generally based on the different works[2,18,42,45,46],promotion of cobalt catalysts by noble metals such as Ru,Re and Pt leads to intensify the cobalt oxide reduction.Parnian et al.[18]showed that loading of Ru in the Co/γ-Al2O3catalysts presented an influential factor on the first reduction peak so that the second peak did not vary.However,in the present study,the TPR results demonstrated that the Ru promoter had more significant effect on the reduction peak of CoO to Co0than Co3O4to CoO that was in accordance with those given in the previous works[42,45].Hence,the results of the present work are in agreement with those of the literature regarding the influences of promoters,particularly the noble metals for the cobalt catalysts.Moreover,for the catalysts prepared via chemical reduction method,the intensity of the reduction peaks raised with enhancement of the size of cobalt oxide particles as confirmed by XRD and TEM tests that allows the reduction of cobalt be carried out easier by enhancement of Ru loading.Haghtalab and Mosayebi[7]observed that the intensity of the reduction peaks enhanced by increasing the size of the cobalt oxide particle in Co/γ-Al2O3catalysts prepared via surface displacement reaction mechanism.
| 图表编号 | XD0042893900 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.01.01 |
| 作者 | Jafar Shariati、Ali Haghtalab、Amir Mosayebi |
| 绘制单位 | Department of Chemical Engineering, Tarbiat Modares University、Department of Chemical Engineering, Tarbiat Modares University、Department of Chemical Engineering, Tafresh University |
| 更多格式 | 高清、无水印(增值服务) |